Biocatalysists for Industrial and Medical Applications
Submitting Institution
Queen's University BelfastUnit of Assessment
ChemistrySummary Impact Type
TechnologicalResearch Subject Area(s)
Chemical Sciences: Inorganic Chemistry, Organic Chemistry, Physical Chemistry (incl. Structural)
Summary of the impact
Queen's University Belfast has developed a number of biocatalytic
processes for the production of
pharmaceutical intermediates which have been applied commercially. The
most significant
involved Vernakalant, a new drug for treatment of the most common form of
irregular heartbeat,
now available in the EU, and currently awaiting approval in the USA and
Canada. In addition, QUB
has sold £300,000 worth of bioproducts and through the collaborations with
Almac Sciences
facilitated the initiation of their biocatalysis business which currently
is a multi-million revenue
earner for Almac Sciences and employs 30 staff, including 15 PhD graduates
from the Queen's
group.
Underpinning research
The research at Queen's supervised by Boyd (now Emeritus Professor) and
Stevenson has led
to an understanding of the remarkable potential of a range of biocatalysts
which produce
single enantiomer polyoxygenated products from aromatic substrates. From
1993, Boyd, in
collaboration with Dalton (Warwick), who supplied the initial enzymes
(toluene dioxygenase
mutant strains), developed the synthesis of the cis-dihydrodiols
from monosubstituted benzenes
which were used as intermediates for the synthesis of Vernakalant (Figure
1).
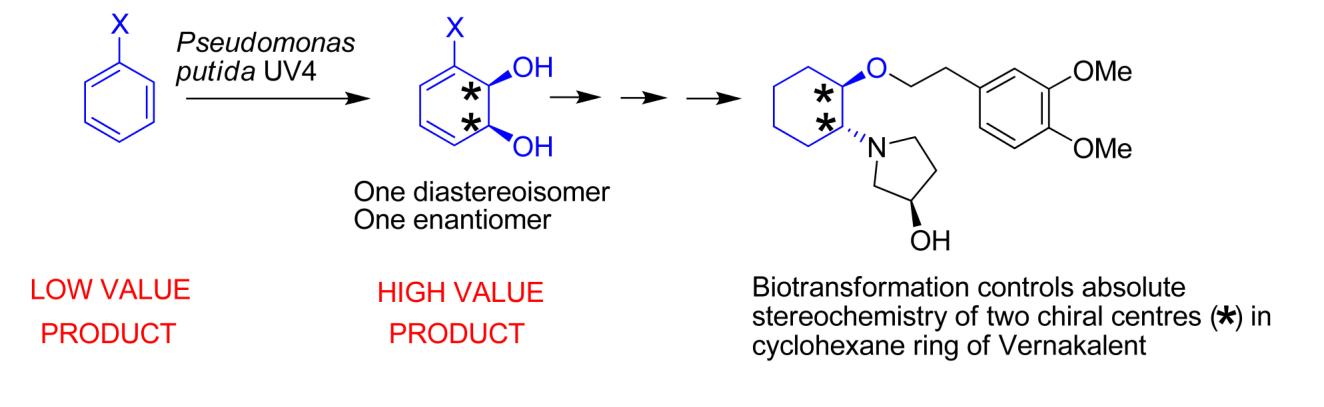 Figure 1 Reaction scheme for the formation of cis-dihydrodiols
from monosubstituted
aromatics leading to the formation of Vernakalant
Figure 1 Reaction scheme for the formation of cis-dihydrodiols
from monosubstituted
aromatics leading to the formation of Vernakalant
Importantly, the ability to translate the fundamental research into a
commercial success, was due
to the Queen's group enabling the determination of structure,
configuration and enantiopurity of
these novel bioproducts (References 1 and 2 in section 3). Through
this knowledge, the scope and
diversity of the bioproducts produced has been expanded significantly and
the limitations of the
substrates able to be biotransformed alleviated by the use of manipulation
of the biocatalyst
employed. The identification of the latter was critical and the Queen's
group led the search for
new enzymes through development of recombinant strains within Biological
Sciences in Queen's
(Kulakov and Allen) as well as with Gibson (University of Iowa) developing
naphthalene and
biphenyl dioxygenases. This research included the first example of
site-directed mutagenesis
being used to modify dioxygenase regio- and stereo-selectivity (Reference
3 in section 3). From
these new catalytic systems, previously unknown families of enantiopure
metabolites with novel
structures, such as sulfoxide diols, arene-derived triols and tetrols,
cyclohexenone cis-diols, arene
oxides and hydrates, with virtually all the products of the biocatalytic
reactions being single
enantiomers, were developed (Reference 4 in section 3).
Furthermore, empirical models
were devised to allow prediction of the preferred products formed and the
absolute configurations
of new bio-products. In addition, this research has contributed to an
understanding of why
polycyclic aromatic hydrocarbon (PAH) pollutants are human carcinogens;
mammalian redox
enzymes (monooxygenases) play a key role in turning PAHs into arene oxide
and diol epoxide
metabolites that interfere with DNA replication.
From the knowledge and fundamental understanding of the biocatalytic
pathways, and the
manipulation of the conditions used for the reaction and separation
processes, as well as
the analytical techniques established, commercialisation of the
bioproducts has been achieved.
This understanding has led to the utilisation of a wide range of
substrates which had previously
been intractable and, therefore, the selective and efficient production of
a large number of
desirable pharmaceuticals and fine chemicals in an economic process.
Applications of its
enantiopure bioproducts have been made in the synthesis of natural
products including
pericosines (see reference 5 in section 3) and epibatidine,
unnatural products, such as
carbasugars and chiral ligands, for example, bipyridines (see reference 6
in section 3),
aminoalcohols and phosphines/phosphine oxides.
References to the research
* signify the references
which best indicate the quality of the underpinning research
*1. Boyd, D. R.; Sharma, N. D.; Barr, S. A.; Dalton, H.; Chima, J.;
Whited, G.; Seemayer,
R., Chemoenzymatic Synthesis of the 2,3-Cis-Dihydrodiol and 3,4-Cis-Dihydrodiol
Enantiomers
of Monosubstituted Benzenes. J. Am. Chem. Soc. 1994, 116, (3), 1147-1148.
(DOI: 10.1021/ja00082a053)
2. Allen, C. C. R.; Boyd, D. R.; Dalton, H.; Sharma, N. D.; Brannigan,
I.; Kerley, N. A.; Sheldrake,
G. N.; Taylor, S. C., Enantioselective bacterial biotransformation routes
to cis-diol metabolites of
monosubstituted benzenes, naphthalene and benzocycloalkenes of either
absolute
configuration. J. Chem. Soc., Chem. Commun. 1995, (2), 117-118. (DOI:
10.1039/c39950000117)
3. Parales, R. E.; Resnick, S. M.; Yu, C. L.; Boyd, D. R.; Sharma, N. D.;
Gibson, D. T.,
Regioselectivity and enantioselectivity of naphthalene dioxygenase during
arene cis-
dihydroxylation: Control by phenylalanine 352 in the alpha subunit. J.
Bacteriol. 2000, 182,
(19), 5495-5504. (DOI: 10.1128/JB.182.19.5495-5504.2000))
*4. Boyd, D. R.; Sharma, N. D.; Malone, J. F.; Allen, C.C.R . New
families of enantiopure
cyclohexenone-cis-diol, o-quinol dimer and hydrate metabolites from
dioxygenase-
catalysed dihydroxylation of phenols, Chem. Commun.,2009, 3633-3635 (DOI:
10.1039/b905940g).
*5. Boyd, D. R.; Sharma, N.D.; Acaru, C.; Malone, J.F.; O'Dowd, C.R.
Allen,; C. C.R.;
Stevenson, P.J., Chemoenzymatic synthesis of carbasugars (+)-pericosines
A-C from diverse
aromatic cis- dihydrodiol precursors, Org. Lett. 2010, 12,
2206-2209 (DOI: 10.1021/ol100525r)
6. Boyd, D. R.; Sharma, N. D.; Sbircea, L. ; Murphy, D. ; Belhocine, T.;
Malone, J. F.; James, S.
L.; Allen C. C. R. ; Hamilton, J. T. G.; Azaarene cis-dihydrodiol-derived
2,2'-bipyridine ligands for
asymmetric allylic oxidation and cyclopropanation, Chem. Commun., 2008,
5535-5537 (DOI:
10.1039/b814678k). See also
http://www.rsc.org/Publishing/Journals/cc/News/B814678K_Boyd_B812366G_James.asp
Details of the impact
Cardiome Pharma, a Canadian pharmaceutical company, recognised in 2003
that the team
at QUB had the ability to deliver kilogram quantities, to GMP standard, of
a chiral
intermediate required for an alternative chemoenzymatic route to their new
drug candidate
RSD1235 for a clinical trial for the treatment of atrial fibrillation by
intravenous injection. Atrial
fibrillation, which is linked with strokes, is the most common form of
irregular heartbeat, with an
estimated nine million sufferers worldwide and established drugs for
restoring normal heart
rhythms are limited by either modest efficacy and/or side effects.
After investing more than CAN $1M prompted by research in two seminal
publications by
the Queen's group (Reference 1, J. Am. Chem. Soc., 1994, 116, 1147;
Reference 2, J.
Chem. Soc., Chem. Commun. 1995, 117 in section 3), during 2004 QUB
was employed to
deliver 5 kg of an enantiopure bioproduct and develop the synthetic
chemistry used in the first
synthetic steps toward RSD1235 (Reference 1 in section 5). Using
this alternative improved
chemoenzymatic route, Cardiome ultimately prepared 1 kg of homochiral
RSD1235 to GMP
standard required for clinical trials based on the material and routes
Queen's provided
(Reference 2 in section 5). As a result of the clinical trials, in
2009 Merck signed a licensing
agreement with Cardiome worth up to 600 million dollars to help rapidly
get the drug to
market (Reference 3 in section 5). RSD 1235 has completed clinical
trials, is now marketed as
Vernakalant and in September 2010 was approved for use in over ten
European countries and
other areas under the trade name Brinavess (Cardiome/Merck). Vernakalant
is currently in
phase 3 clinical trials for FDA approval for use in the USA and is
currently being evaluated by
the National Institute for Health and Clinical Excellence, NICE, as a
prescription drug for NHS
use in the UK (Reference 4 in section 5).
Secondly, Almac Sciences have over the past five years developed an
increasing biocatalytic
capability within their business facilitated by the research undertaken in
Queen's led by Boyd
and Stevenson. Many of the initial bioproducts marketed by Almac
SelectAZyme, and new
projects undertaken for external customers, were solely based on the
biocatalytic pathways,
enzymes, expertise and facilities used for the formation of polyoxygenated
cyclohexanes from
aromatics developed in Queen's which are utilized as precursors in
synthetic routes to
bioactive materials, such as the influenza drug Tamiflu (Reference 5 in
section 5). The
development of the routes for commericalisation was undertaken through a
collaboration between
the two groups of researchers (Reference 6 in section 5). This
area is now growing into other
markets significantly and the biocatalysis group in Almac now employ 30
staff including 15 PhD
graduates from QUB trained in Boyd and Stevenson's groups. The
biocatalysis group operates as
a multi-million revenue provider for Almac providing solutions to
customers through the application
of enzymes.
Therefore, this biocatalysis research has led to a number of economic and
health related
impacts whose beneficiaries are primarily the industrial partners and the
resultant economic
benefits to the general public as well as the new development of
treatments used in general
practice worldwide.
Sources to corroborate the impact
1: Letter of support from Cardiome:by Senior Director, Research
(Chemistry)
"The route employing the cis-dihydrodiol as starting material was
sufficiently promising that
the process was optimized and demonstrated in the production of 1kg of
cGMP vernakalant
starting from 5kg of the cis-diol produced by Professor Boyd and
his colleague Chris Allen".
2: The Cardiome request for chlorobenzene cis-dihydrodiol (5kg
fermentation product) for
1kg GMP manufacture of RSD1235 by Raylo and a possible advantage of the
new
chemoenzymatic route based on an earlier (1 kg) delivery :-
"Not only has the new route of manufacture for RSD1235 been shown to be
feasible on tens
of gram scale by Raylo, but a significant improvement in yield from 40% to
65-70% for the last
critical displacement step has been made by Raylo and Medichem."
3: Merck & Co. announces $600-million licensing agreement for
Cardiome's vernakalant (www.firstwordpharma.com/node/359114)
4: NICE — Health Technology Appraisal for Vernakalant for the treatment
of recent onset
atrial fibrillation (http://guidance.nice.org.uk/TA/Wave26/7/DraftScope/pdf/English)
5: Almac.SelectAZyme brochure offering a new range of single enantiomer
substituted
benzenecis-diols.
6: Letter of support from Almac Sciences Ltd by Head of Biocatalysis
& Isotope Chemistry.