Case 3 – Ultra-Low-Power Electronics for Healthcare Applications
Submitting Institution
Imperial College LondonUnit of Assessment
Electrical and Electronic Engineering, Metallurgy and MaterialsSummary Impact Type
TechnologicalResearch Subject Area(s)
Engineering: Electrical and Electronic Engineering
Technology: Communications Technologies
Summary of the impact
Professor Chris Toumazou FRS and his team at Imperial College have
developed biomedical technologies based on ultra-low-power CMOS and ISFET
electronics to provide the medical community with the means to rapidly
diagnose, monitor, and treat diseases with confidence and at low cost.
Since 2008, the impact of this research has been to:
I1) spinout a start-up company DNA Electronics (DNAe) to deliver
point-of-care products to quickly recognize genetic diseases and identify
potential poor drug interactions;
I2) enable Life Technologies (formerly Ion Torrent) to develop the
Personal Genome Machine (PGM) that have generated $100m in sales (in the
18 months since its launch) using DNAe's core semiconductor sequencing IP;
I3) save lives by using the PGM in clinical and public health
applications;
I4) spinout a second start-up company (Toumaz) that has released
SensiumVitals®, a FDA-approved and CE-marked ultra-low power system for
wireless monitoring of patient vital signs;
I5) provide early warning of adverse physiological events in clinical
settings using the SensiumVitals® platform resulting in improved quality
of patient care and reduced demand on intensive care provision in
hospitals internationally.
Underpinning research
Over the last decade we have seen a convergence of semiconductor
technology into the world of healthcare, providing new solutions for early
detection, diagnosis and therapy of disease. Disruptive semiconductor
technology in healthcare relies upon leveraging CMOS technology in novel
and unusual ways, for example, designing custom VLSI structures for
biosensing, or by exploiting the underlying device physics for energy
efficient low precision analogue processing. The biomedical electronics
team at Imperial led by Professor Christofer Toumazou has produced key
advances in this area as described below.
There are two aspects to the underpinning research for this case study.
The first relates to point-of-care diagnostics for the detection of
genetic sequences. This field requires biosensing platforms that are
sensitive to the target sequence, are fast, can be mass-manufactured, and
are disposable. Conventional lab-based methods of detecting DNA sequences
rely on optical methods; typically by the addition of fluorescent tags to
the target DNA that in turn latches onto a DNA probe sequence only if
there is a match between the two. Our research this past decade has
developed a pioneering all-electrical approach that does not require
tagging of DNA. The principle of label-free electrochemical DNA
detection using an ion-sensitive field effect transistor (ISFET) was
originally described in [R1]. This is now referred to in the industry as
"next generation semiconductor sequencing".
A problem with electro-chemical sensors is that they are known to suffer
from non-idealities and significant variability in parameters such as
drift, offset, sensitivity and lifetime. Fabricating them in CMOS makes
them cheap, easy to mass-produce, and allows integration with electronic
circuits, but at the same time further degrades their performance. The
breakthrough of our research came from the recognition that such on-chip
sensors, although inaccurate for absolute measurement, could be harnessed
for relative monitoring, i.e. detecting changes rather than absolute
values. Specifically, we engineered methods for achieving robust
on-chip relative pH sensing based on the Ion-Sensitive Field Effect
Transistor (ISFET) in a standard CMOS technology. Through a variety
of device, circuit and system innovations, a platform that is low cost,
easy to manufacture and reliable has been realized [R2, R3].
The second aspect of the underpinning research has been the reduction of
transmitted bandwidth from biosensors. Sensory systems acting on real
world data typically consist of an analogue sensor interface with signal
conditioning, data conversion, and wireless communication, requiring high
bandwidth when transmitting raw data. Our research established how to
achieve power reduction by placing local intelligence at the front-end,
thus reducing communication bandwidth. Using analogue signal processing
prior to data conversion, ultra energy-efficient processing could be
achieved but at the expense of variability (i.e. drift) and
reconfigurability. These limitations were overcome by combining
closed-loop digital calibration to ultra low power analogue circuits thus
achieving both energy efficiency and precision [R4, R5]. This ultra low
power approach has been demonstrated in a number of applications including
the world's first totally implantable cochlear prosthesis. This new
prosthesis was independently assessed to reduce power consumption by up to
two orders of magnitude compared to the state-of-the-art [R6].
References to the research
(*References that best indicate quality of underpinning research.)
[R1]* S. Purushothaman, C. Toumazou, J. Georgiou, "Towards
fast solid state DNA sequencing", IEEE International Symposium on
Circuits and Systems, (2002), 4, pp.169-172. DOI: 10.1109/ISCAS.2002.1010416
[R2]* L. Shepherd, C. Toumazou, "A Biochemical Translinear
Principle with Weak Inversion ISFETs", IEEE Transactions on Circuits
and Systems I: Regular Papers, (2005), 52(12), pp. 2614-2619.DOI: 10.1109/TCSI.2005.857919
[R3] L. Shepherd, P. Georgiou, C. Toumazou, "A novel
voltage-clamped CMOS ISFET sensor interface", IEEE International
Symposium on Circuits and Systems (ISCAS 2007), 27-30 pg 3331 - 3334 May
2007. DOI: 10.1109/ISCAS.2007.378224
[R4] O. Omeni, C. Toumazou, "A CMOS micro-power wideband
data/power transfer system for biomedical implants", IEEE
International Symposium on Circuits and Systems (ISCAS 2003), 25-28 Vol 5,
V-61 — V-64 May 2003. DOI: 10.1109/ISCAS.2003.1206184
[R5] O. Omeni, E. Rodriguez-Villegas, C. Toumazou, "A
micropower CMOS continuous-time filter with on-chip automatic tuning",
IEEE Transactions on Circuits and Systems I: Regular Papers, (2005),
52(4), pp.695-705.DOI: 10.1109/TCSI.2005.844112
[R6]* J. Georgiou, C. Toumazou, "A 126-03bcW cochlear chip for
a totally implantable system", IEEE Journal of Solid-State Circuits,
(2005), 40 (2), pp.430-443.DOI: 10.1109/JSSC.2004.840959
Details of the impact
We now provide details of the 5 aforementioned impacts and their links to
underpinning research:
I1) DNA Electronics (DNAe) is a start-up company founded by
Toumazou to exploit the group's breakthrough in semiconductor-based gene
sequencing in [R1, R2, R3]. Since 2008, DNAe has developed two genetic
analysis platforms: a DNA sequencing platform suited for laboratory use,
and a "point-of-care" platform called Genalysis® which is suited for
rapid, lab-free testing by non-expert users [E1].
DNAe now employs over 45 full time staff in the UK, brought in revenue of
in excess of £25 million through their licensing and collaboration
activity, and won six industrial awards. These include: the BBC Focus
award for Innovation, the Elektra European Electronics Industry Award for
R&D, and e-Legacy Award for Medical Advances (all in 2009); and three
IET Awards for Healthcare, for Emerging Technologies and for Electronics
(all in 2010).
I2) DNAe's sequencing platform technology with its associated patents
(US7888015, US 7649358, US7686929, US8114591), all based on the
underpinning research [R1-R3], was licensed to Ion Torrent Systems [E2]
and Roche in 2010 [E3]. Ion Torrent Systems was subsequently bought by
Life Technologies Inc. for $725 million. Their first DNA sequencing
product based on DNAe's technology [E4], the Personal Genome Machine
(PGM), was launched in Q1 2011. In the first 18 months since launch in the
first quarter of 2011, 1300 units of the PGM were shipped and generated
around $100 million in sales revenue for Ion Torrent [E5]. The fact that
the PGM is based on DNAe's technology is corroborated in [E4], stating:
"Ion semiconductor sequencing is based on the detection of hydrogen
ions released during DNA synthesis. Ion Torrent licensed the technology
from DNA Electronics in 2010 and incorporated it into products such as
the Ion Personal Genome Machine (PGM)."
I3) Since its release in 2011, the PGM product is being used for clinical
and public health applications, and lives have been saved as a result. For
instance, the Beery twins' rare genetic disease, dopa-responsive dystonia,
was discovered by DNA sequencing using our technology and has since been
treated with appropriate medication [E6]. Another example of the impact of
our DNA sequencing technology is demonstrated in combating the German E.
Coli outbreak of 2011, which was so serious that at least 26 people died
and more than 2,700 have been sickened by the outbreak in at least 13
different countries [E7]. Using the PGM, scientists were able to complete
rapidly the process of sequencing the 5.4 million letters of the latest
bug's genetic code in only three days [E7]. Previous light beam based DNA
sequencers would have taken at least a week to read the code of an
organism's genome.
I4) Toumaz is a second start-up; founded to exploit the group's
research in ultra-low power techniques for wireless physiological
monitoring. Since 2008, Toumaz developed the SensiumVitals® platform, a
wireless system designed to monitor the vital signs of hospital patients.
The system, which exploits the underpinning research in [R4, R5 and R6],
comprises of a lightweight disposable wireless patch that monitors a
patient's vital signs (heart-rate, respiration and axillary temperature)
and then wirelessly communicates this data to doctors and nurses via the
hospital's patient monitoring IT system. The SensiumVitals® digital
plaster is the first product aimed at the general floor population of a
hospital to have received FDA (510k) approval in 2011 [E8] and
subsequently received the CE mark in 2013.
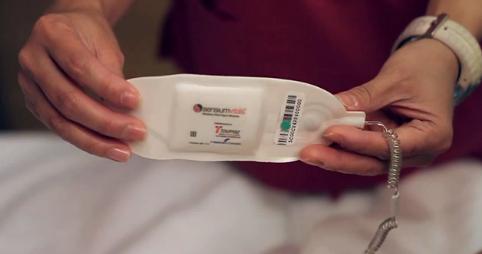 Toumaz's FDA approved and CE marked SensiumVitals® digital plaster.
Toumaz's FDA approved and CE marked SensiumVitals® digital plaster.
Toumaz is now an AIM-listed company (symbol: TMZ) employing 180 people in
five countries (of which approximately half are in the UK), with 2012
year-end assets of GBP 51.9 million, and revenue (pro-forma) of GBP 22.3
million. Toumaz has won numerous awards prior to 2008 and the National
Microelectronics Institute (NMI) award in 2009.
I5) The Toumaz SensiumVitals® digital plaster has proven to be beneficial in
a clinical setting. In a trial at Saint John's Health Centre in Santa
Monica, CA, it was demonstrated that the SensiumVitals® system provided
early warnings of adverse physiological events to clinical staff. The study
showed that the system enables early detection of deterioration, meaning
patients can be treated more quickly and safely. In addition, the device was
shown to be better than alternatives for patient recovery because it is
lightweight, unobtrusive, and comfortable to wear, and so enables patients
to walk around. The system was also shown to integrate well with existing
clinical workflows. The development of this new technology is widely
believed to be part of a new revolution in healthcare technology [E9].
Toumaz has secured an international distribution agreement for its
SensiumVitals® digital plaster from NantHealth Group in the US in Oct 2013.
Through an initial order of 250,000 units, the SensiumVitals® digital
plaster is being fully adopted first in Accident & Emergency at Hurley
Medical Center in Flint, Michigan (US) [E10].
In recognition of Professor Toumazou's success ".... in applying
semiconductor technology to biomedical and life-science applications,
most recently to DNA analysis", Royal Society awarded him the 2013
Gabor Medal (http://royalsociety.org/awards/gabor-medal/).
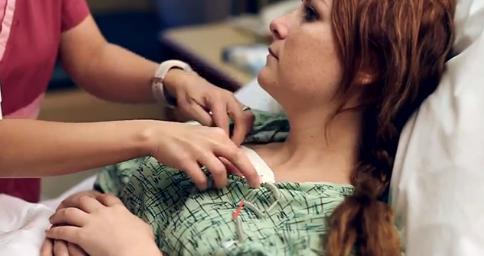 Toumaz SensiumVitals® digital plaster in trials at Saint John's Health
Centre (Santa Monica, CA) — the http://www.youtube.com/watch?v=uVxO4xh_dDs
Toumaz SensiumVitals® digital plaster in trials at Saint John's Health
Centre (Santa Monica, CA) — the http://www.youtube.com/watch?v=uVxO4xh_dDs
Sources to corroborate the impact
E1) "Powering Preventative Medicine", Ken Davies, 27 Sept 2011,
Bio IT World. http://www.bio-
itworld.com/issues/2011/sept-oct/powering-preventative-medicine.html
or https://www.imperial.ac.uk/ref/webarchive/8hf
(archived on 26/4/13)
E2) "DNA Electronics Licenses IP to Ion Torrent", GenomeWeb Daily News, 3
August 2010. http://www.genomeweb.com/sequencing/dna-electronics-licenses-ip-ion-torrent.
Archived here
on 23/10/2013.
E3) "Roche, DNA Electronics Partner on Semiconductor-based Sequencing",
GenomeWeb Daily News, 1 Nov 2010. http://www.genomeweb.com/sequencing/roche-dna-electronics-partner-semiconductor-based-sequencing.
Archived
here on 23/10/2013.
E4) "Advances in DNA sequencing lead to patent disputes", Nature
Biotechnology, Vol. 30, pp. 1054-1058, 8 Nov. 2012.
http://www.nature.com/nbt/journal/v30/n11/full/nbt.2407.html?WT.ec_id=NBT-201211
DOI: 10.1038/nbt.2407
E5) "Analyst: The Better Desktop DNA Sequencer May be Losing The
Marketing War", Matthew Herper, 15 Aug 2012, Forbes. http://www.forbes.com/sites/matthewherper/2012/08/15/analyst-
the-better-desktop-dna-sequencer-may-be-losing-the-marketing-war/ or
https://www.imperial.ac.uk/ref/webarchive/fkf
(archived on 29/4/13).
E6) "Twins thank Biotechnology for the Quality of Their Lives", Darleen
Hartley, 2 Feb 2012, Bright Side of News. http://www.brightsideofnews.com/news/2012/2/2/twins-thank-biotechnology-for-
the-quality-of-their-lives.aspx or https://www.imperial.ac.uk/ref/webarchive/9hf
(archived on 26/4/13).
E7) "Gene Probe Yields E. coli Clue", Gautam Naik and Laura Stevens, 9
June 2011, The Wall Street Journal.
http://online.wsj.com/article/SB10001424052702304778304576373650243960700.html
or https://www.imperial.ac.uk/ref/webarchive/h8f
(archived on 18/11/13).
E8) "Toumaz forms joint venture to commercialise its Sensium Digital
Plaster", Julien Happich, 12 July 2011, EE Times. http://www.electronics-eetimes.com/en/toumaz-forms-joint-venture-to-commercialise-its-sensium-digital-plaster.html?cmp_id=7&news_id=222908280
or
https://www.imperial.ac.uk/ref/webarchive/kjf
(archived on 26/4/13).
E9) "Towards the improvement of patients' safety
with continuous wireless monitoring: Pilot at Saint John's Health Center",
Toumaz Group white paper, 2013.
http://www.toumaz.com/sites/default/files/White%20paper%20-%20v1%206%20NL.pdf
Archived here
on 23/10/2013.
E10) "Distribution agreement: first commercial US orders", Press Release,
15 Oct 2013. http://www.investegate.co.uk/ArticlePrint.aspx?id=201310150700174835Q
or https://www.imperial.ac.uk/ref/webarchive/twf
(archived on 16/10/13).