3 Elemental Fluorine for Fine Chemical Manufacture
Submitting Institution
University of DurhamUnit of Assessment
ChemistrySummary Impact Type
TechnologicalResearch Subject Area(s)
Chemical Sciences: Organic Chemistry, Physical Chemistry (incl. Structural), Other Chemical Sciences
Summary of the impact
Durham selective direct fluorination methodology using fluorine gas has
been scaled up by F2
Chemicals Ltd to supply the Pfizer company with multi-tonne quantities of
a key pharmaceutical
intermediate used in the synthesis of V-Fend (voriconazole). This
antifungal agent has achieved
global sales of $4.65bn from 2008-present and is used extensively for the
treatment of invasive
pulmonary aspergillosis. Multi-channel continuous flow gas/liquid
microreactor technology for direct
fluorination was licensed to the Asahi Glass Co (Japan) and other
transformations enabled by
fluorine gas are being exploited by a DU spin-out company, Brock Fine
Chemicals Ltd.
Underpinning research
Research in elemental fluorine for organic synthesis at DU was led by
Prof R.D. Chambers FRS
(Durham staff 1960-2000) and continued by Prof G Sandford (Durham staff
1993-present).
Elemental fluorine gas (F2) has long been considered to be too
reactive and uncontrollable for use
as a reagent in organic synthesis and this perception still predominates.
General comments in
standard organic chemistry textbooks such as "Direct fluorination of
aromatic rings with F2 is not
feasible at room temperature because of the extreme reactivity of F2....is
not yet of preparative
significance (J. March, Advanced Organic Chemistry)" are typical.
Despite this background, research into the use of F2 for
controlled organic synthesis began a new
phase in 1993 after encouragement from Durham led British Nuclear Fuels
(BNFL) to exploit its
expertise in handling F2 for non-nuclear purposes and to create
a subsidiary company, BNFL
Fluorochemicals Ltd (Preston, UK), later F2 Chemicals Ltd. Considerable
research funding from
the company to Durham allowed the development of a wide ranging blue-skies
research
programme into the use of F2 for organic synthesis and this
continues at Durham to the present.
Expertise was developed to overcome the many problems of using F2
for the safe synthesis of fine
chemicals. In particular, techniques involving the use of dilute F2
in nitrogen, appropriate solvent
choice (high dielectric constant media such as formic acid, sulfuric acid
or acetonitrile) [1], reactor
vessel design, gas flow regulator systems and stainless steel/monel
fluorine gas handling lines
have been developed over the years in Durham. This has allowed selective
direct fluorination of a
range of aliphatic, dicarbonyl [2], aromatic, heteroaromatic,
heterocyclic, steroid and carbohydrate
derivatives to be established and the mechanism (regiochemistry,
stereochemistry, selectivity) of
these processes to be explored. Indeed, we have shown that controlled
direct fluorination of
aromatic rings is now feasible at room temperature [1].
The control of F2 reactivity by promoting selective
electrophilic reactions using high dielectric
constant media [1] was particularly important, and F2 can now
be considered to act as a typical
electrophilic reagent for a range of electrophilic aliphatic and aromatic
substitution processes. In
particular, efficient direct selective fluorination processes of
β-dicarbonyl and β-ketoester
substrates were established for the first time using acetonitrile or
formic acid as reaction media [2]
to give various fluoro-dicarbonyl and fluoro-ketoester systems in high
yield.
Further control of selective fluorination reactions was achieved by the
design, fabrication and
commissioning of single and multi-channel continuous flow reactor systems,
establishing the use of
convenient and inexpensive flow reactors for gas/liquid processes in the
laboratory [3]. Key new
techniques for the supply of individual gas (F2) and liquid
reagents from single sources to a parallel
array of many flow channels at the same flow rate and pressure whilst
maintaining laminar flow
within the reactor channels were incorporated into the reactor designs.
Fluorine gas can also be used an enabler of other chemical
transformations. For example, reaction
of fluorine in situ with iodine leads to iodine monofluoride which
has been used in highly efficient
electrophilic iodination processes [4] within acidic reaction media for
the synthesis of iodoaromatic
systems directly from corresponding aromatic substrates.
References to the research
[1] R. D. Chambers, C. J. Skinner, J. Hutchinson and J. Thomson,
Synthesis of fluoroaromatic
compounds. J. Chem. Soc., Perkin Trans. I, 1996, 605-609. DOI:
10.1039/P19960000605.
[27 citations]
[2] R. D. Chambers, M.P. Greenhall and J. Hutchinson. Direct fluorination
of 1,3-dicarbonyl
compounds. Tetrahedron, 1996, 1-8. DOI:
10.1016/0040-4020(95)00883-A [57]
[4] R.D. Chambers, C.J. Skinner, M.J. Atherton and J.S. Moilliet. Use of
elemental fluorine for
the halogenation of aromatics. J. Chem. Soc., Perkin Trans 1,
1996, 1659-1664. DOI:
10.1039/P19960001659. [17]
The research quality of the elemental fluorine research programme
(1993-present) led by Prof RD
Chambers (RDC) and Prof G Sandford (GS) is supported by: RDC's election to
FRS in 1997; and
the 2003 award of the Prix Moissan to RDC in 2003 (the premier
international award in Fluorine
Chemistry). GS and RDC have given many plenary and keynote lectures at
major international
conferences (European Symposium on Fluorine Chemistry, ACS Winter Fluorine
Symposium, ACS
National Meetings and International Symposium on Fluorine Chemistry). R.D.
Chambers, Fluorine
in Organic Chemistry (1st edition: 1973; 2nd
edition: 2004) remains the standard textbook in the
field.
Research funding allowing the fluorination programme to be established
included: industrial
support from BNFL Fluorochemicals (three employees seconded to Durham for
3 years; 3 year
PDRA; 2 PhD studentships); the Asahi Glass Co. Japan (2 PhD studentships
including one
employee from Japan seconded to Durham); and the Royal Society (URF to GS,
1996-2001). The
development of single and multi-channel microreactors was funded by: EPSRC
ROPA (1 PDRA, 3
years) and EPSRC Crystal Faraday (1 PDRA, 3 years) schemes, both in
collaboration with F2
Chemicals.
Details of the impact
In 1992 BNFL established a spin-out company, BNFL Fluorochemicals Ltd,
later F2 Chemicals Ltd,
to develop new markets in the fine chemicals sector using their expertise
in the production and
handling of F2 developed from nuclear power generation
applications. A research team (3 PDRA
employees, 3 years) from the company was seconded to the Chemistry
Department at Durham
(1992-1995) in order to establish a skill base, expertise and IPR in the
field. The company also
provided funds for building and equipping a new, purpose-built research
laboratory for handling F2
within the Chemistry Department and RDC subsequently became a
non-executive Director of the
company. The following period (1993-1996) resulted in a suite of over 20
patent applications which
were filed, granted and maintained by F2 Chemicals arising from the Durham
research
collaboration. Most importantly, development of new selective fluorination
methodology of β-ketoesters
in high dielectric constant media was investigated and exemplified at
Durham on a 1 g
scale and published by the DU research team in collaboration with F2
Chemicals in 1996 [2]
following IPR protection [Im1]. Subsequently, Durham direct fluorination
reaction methodology
using F2 [2] was adopted and scaled up to a manufacturing
process by F2 Chemicals Ltd [Im2] with
the design, investment and construction of a 1000 litre Selective Direct
Fluorination (SDF) plant
(Fig. 1a) at their headquarters in Preston to synthesise products for
customers in the life science
industries.
V-FEND (Voriconazole, Pfizer, Fig. 1b) is the world-wide best-selling
systemic, antifungal agent
and has a 5-fluoropyrimidine sub-unit 1 as part of its structure.
Manufacture of fluoropyrimidine
intermediate 1 had been carried out previously by multi-step,
resource intensive strategies
described by Pfizer scientists [Im3] but new Durham methods for selective
direct fluorination of β-ketoesters
using F2 [2] provided the opportunity for a more efficient
2-step process, that is far less
expensive and generates less waste than other procedures. Given this new
business opportunity,
Durham direct fluorination methodology [2] for the synthesis of
β-fluoroketoester 2 (Fig. 1b), as the
key starting material for the manufacture of 5-fluoropyrimiide system 1,
was developed and scaled-up
by F2 Chemicals and used through all the clinical trial, launch and
commercialization periods of
V-FEND by Pfizer [Im3]. In the period from January 2008 to July 2013,
multi-tonne quantities of F-ketoester
1 were manufactured by F2 Chemicals Ltd [Im2] as the exclusive
supplier for Pfizer using
Durham direct fluorination chemistry [2]. World-wide sales of V-FEND in
the 2008-2012 REF period
total $4.65 billion [Im4] making this product one of the global top 100
best-selling pharmaceuticals.
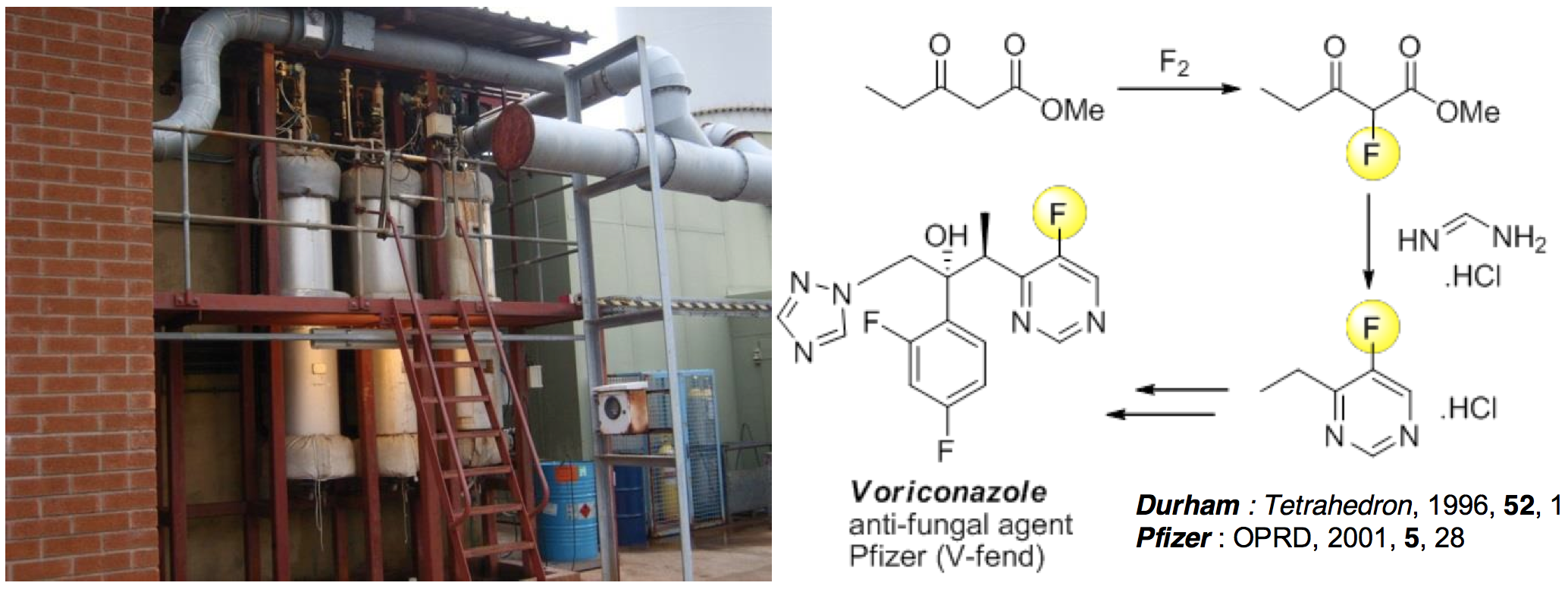 Figure: impact of Durham selective direct fluorination methodology. Left: Selective Direct Fluorination plant at F2 Chemicals (Preston). Right: new strategy for the synthesis of a fluoro-ketoester, a key intermediate of Pfizer’s V-Fend antifungal agent.
Figure: impact of Durham selective direct fluorination methodology. Left: Selective Direct Fluorination plant at F2 Chemicals (Preston). Right: new strategy for the synthesis of a fluoro-ketoester, a key intermediate of Pfizer’s V-Fend antifungal agent.
V-FEND's economic and societal impact arises from its use as a triazole
antifungal medication
[Im5] active against serious, invasive fungal infections such as
candidiasis, aspergillosis, and
certain emerging fungal infections [Im6]. Aspergillosis is primarily an
infection of the lungs caused
by the inhalation of airborne spores of the fungus Aspergillus
which is commonly found growing on
dead leaves, stored grain, compost piles, or in other decaying vegetation.
There are several forms
of aspergillosis: pulmonary aspergillosis is an allergic reaction to the
fungus that usually develops
in people who already have lung problems (such as asthma or cystic
fibrosis); aspergilloma is a
growth (fungus ball) that develops in an area of past lung disease or lung
scarring (such as
tuberculosis or lung abscess) and pulmonary aspergillosis (invasive type)
is a serious infection
associated with pneumonia that can spread to other parts of the body. This
infection almost always
occurs in people with a weakened immune system due to cancer, AIDS,
leukaemia, an organ
transplant, chemotherapy, or other conditions or medications that lower
the number of normal
white blood cells or weaken the immune system. For example, invasive
pulmonary aspergillosis
(IPA) is estimated to occur in 5-13% of people who have a bone marrow
transplant, 5-25% of
people with a heart or lung transplant and 10-20% of people who undergo
high-dose radiotherapy
for leukaemia.
Durham selective fluorination chemistry [2, Im1] has therefore played a
significant role in impacting
many patients treated by V-FEND, giving world-wide health benefits in the
treatment of fungal
infections for a wide range of disease control.
In order to further develop the use of Durham F2 chemistry for
fine chemical manufacture, a
Durham University spin-out company, Brock Fine Chemicals Ltd [Im7], was
established in April
2011 by Graham Sandford with assistance and legal expertise from Durham
Business Innovation
Services (DBIS). Brock (UK registered company 7610103) attracted proof of
concept funding
(£100K) from the NorthStar regional investment group [Im8] to further
exploit the use of fluorine for
fine chemical manufacturing, particularly for the synthesis of a range of
iodo-aromatic derivatives
using Durham fluorine-mediated iodination chemistry [4]. The company now
employs 2 FTE
chemists and associated marketing and finance expertise. It has made sales
of over 100 fine
chemical products to chemical distributors such as Fluorochem, Acros, Alfa
Aesar, Apollo Scientific
and Shigematsu since trading began. Sales in Year 1 were £8K growing to
£40K in Year 2 and
within the proof-of-concept business plan.
Multi-channel continuous flow microreactor techniques developed at Durham
[3] were patented by
Durham University [Im9] and a world-wide exclusive license negotiated by
the University (DBIS)
and granted to the Asahi Glass Co., Japan for a significant fee and a
subsequent royalty stream.
This acquisition formed a core part of the IP knowledge base in flow
reactor technology at Asahi
Glass.
Sources to corroborate the impact
[Im1] Fluorination of β-ketoesters patent: R.D. Chambers, M.P.
Greenhall, J. Hutchinson, J.S.
Moilliet, J. Thomson, PCT Intl Appl WO 95/14646 (June 1st
1995); Chem. Abstr. 1995, 123,
339705.
[Im2] F2 Chemicals: Managing Director, F2 Chemicals Ltd, www.f2chemicals.com.
[Im3] V-FEND application: the use of Durham/F2 Chemicals direct
fluorination methods for the
synthesis of V-FEND is described by Pfizer scientists in M. Butters and
co-workers, Org.
Proc. Res. Dev., 2001, 5, 28-36.
[Im4] V-FEND sales: annual global sales of V-FEND are given in successive
Pfizer Annual reports:
2008: http://www.pfizer.com/files/annualreport/2008/financial/financial2008.pdf
(p 2)
2009: http://www.pfizer.com/files/annualreport/2009/financial/financial2009.pdf
(p 21)
2010: http://www.pfizer.com/files/annualreport/2010/financial/financial2010.pdf
(p 25)
2011: http://www.pfizer.com/files/annualreport/2011/financial/financial2011.pdf
(p 21)
2012: http://www.pfizer.com/files/annualreport/2012/financial/financial2012.pdf (p115)
[Im5] V-FEND (Pfizer): trade name of Voriconazole: http://en.wikipedia.org/wiki/Voriconazole;
http://www.pfizer.com/products/rx/rx_product_vfend.jsp.
[Im6] V-FEND applications: for details on the various types of
aspergillosis and treatment regimes,
see: http://www.nhs.uk/conditions/Aspergillosis/Pages/Introduction.aspx.
[Im7] Brock Fine Chemicals Ltd: UK registered company 7610103, April 19th
2011,
www.brockfinechemicals.com; sales
figures contained in Annual Reports registered with
Companies House.
[Im8] Brock investment: investment analyst, NorthStar http://www.northstarei.com.
[Im9] Flow systems: multi-channel microreactors patented by DU: R.D.
Chambers, G. Sandford
and D. Holling, U.K. Pat Appl. 0210809.0, 11th May 2002.