UOA04-07: Paracetamol Self-Poisoning: Long-term Effect of Reducing Pack Sizes on Suicides
Submitting Institution
University of OxfordUnit of Assessment
Psychology, Psychiatry and NeuroscienceSummary Impact Type
HealthResearch Subject Area(s)
Medical and Health Sciences: Clinical Sciences, Public Health and Health Services
Summary of the impact
Paracetamol self-poisoning is a major cause of liver failure and death.
Research by Professor Keith Hawton and colleagues in Oxford in the 1990s
revealed the extent and characteristics of the problem, and led to UK
legislation to restrict pack size in 1998. Hawton and colleagues then
showed that this was followed by a substantial reduction, over 30%, in the
number of deaths from paracetamol poisoning. Importantly, a 2013 analysis
shows that the benefit has been sustained and is not diminishing, with an
estimated 374 fewer deaths in the UK since 2008. Registrations for liver
transplants due to paracetamol toxicity have also decreased. As a result
of these benefits, three other countries have introduced similar
restrictions since 2008.
Underpinning research
The characteristics of paracetamol self-poisoning and the potential to
reduce the harms
From 1993, Hawton and his group conducted research into paracetamol
overdoses in the UK, and the circumstances in which they occurred. The
results underpinned the Committee on Safety of Medicine's (CSM) decision
to introduce legislation in 1998 to reduce pack sizes of paracetamol. This
research-driven change in regulations has had continuing benefits in terms
of reducing suicides from paracetamol, and is leading other countries to
make similar restrictions. The research comprised several studies (key
references are cited in Section 3):
Hawton's group showed that the extent of self-poisoning with paracetamol
in the UK over a 10-20 year period reflected its availability, and that
the rate of paracetamol overdose, including fatalities (200-250 per year)
had risen in parallel with sales figures (Gunnell et al., 1997). The team
also reported that 20-30 liver transplants per year in England and Wales
were for paracetamol-induced hepatotoxicity. They furthermore highlighted
the fact that case fatality of paracetamol overdoses was lower in France,
where pack sizes of paracetamol were smaller than in the UK.
In an interview study of 80 patients who took paracetamol overdoses in
1992-3, Hawton and colleagues showed that the ready availability of
paracetamol was the main reason for choosing it for self-poisoning. The
overdoses were very often taken impulsively, and six out of ten used
tablets already available in the household (Hawton et al., 1995). As a
result of this research the team recommended restricting pack sizes of
paracetamol as a means of reducing the number of tablets taken in
overdoses and hence the danger of liver damage and death (Hawton et al.,
1996).
In 1996-7, the Medicines and Health Products Regulatory Agency (MHRA)
reviewed the available evidence on paracetamol self-poisoning. Hawton was
a member of the review group. As stated in a supporting letter (Section 5,
source 2), his research findings were `pivotal' to the outcome of
the review: it recommended to the CSM that, from September 1998, the
maximum pack sizes of paracetamol sold over the counter in pharmacies be
reduced from 100 tablets to 32, and in non-pharmacy outlets from 32 to 16
tablets, with only one pack per transaction. The rationale was that even
if purchasers bought paracetamol more often, the average amount available
in households would be greatly reduced (and hence less available for an
impulsive overdose).
Research showing the sustained benefits of the change in legislation
Hawton's group has also researched the impact of the 1998 change in
legislation. In two initial evaluations, reported in 2001, and in 2004
(Hawton et al., 2004), the group showed that the change had been
beneficial in terms of deaths due to poisoning by paracetamol, the size of
paracetamol overdoses (i.e. how many tablets were taken), and
registrations for liver transplants.
In 2013, the team published a further study and showed the long-term
benefits of the legislation, including a continuing and sustained
reduction in deaths from paracetamol overdose (Hawton et al., 2013),
described in Section 4.
References to the research
Hawton K, Ware C, Mistry H, Weitzel H, Hewitt J, Kingsbury S, Roberts D
(1995) Why patients choose paracetamol for self-poisoning and their
knowledge of its dangers. British Medical Journal 310: 164.
Demonstrated that people taking paracetamol overdoses usually did so
impulsively and often used supplies available in their households.
69 citations (Scopus, accessed 6/10/13).
Hawton K, Ware C, Mistry H, Hewitt J, Kingsbury S, Roberts D, Weitzel H
(1996) Paracetamol self-poisoning: characteristics, prevention and harm
reduction. British Journal of Psychiatry 168:43-48.
Recommended reduced pack sizes of paracetamol as strategy for reducing
risk of a fatal outcome of overdoses. 85 citations.
Hawton K, Fagg J, Simkin S, Bale E, Bond A (1997) Trends in deliberate
self-harm in Oxford 1985-1995: implications for clinical services and the
prevention of suicide. British Journal of Psychiatry 171: 556-560.
Highlighted size of problem of paracetamol overdoses in the UK. 198
citations.
Gunnell D, Hawton K, Murray V, Garnier R, Bismuth C, Fagg J, Simkin S
(1997) Use of paracetamol for suicide and non-fatal poisoning in the UK
and France: are restrictions on availability justified? Journal of
Epidemiology and Community Health 51: 175-179.
Showed that extent of overdoses of paracetamol in the UK were
correlated with sales figures, and fewer deaths from paracetamol
overdose in France where packs were smaller. 85 citations.
Hawton K, Simkin S, Deeks J, Cooper J, Johnston A, Waters K, Arundel M,
Bernal W, Gunson B, Hudson M, Suri D, Simpson K (2004) UK legislation on
analgesic packs: before and after study of long term effect on poisonings.
British Medical Journal (BMJ) 329: 1076-1079.
Demonstrated impact of legislation on paracetamol overdoses, liver unit
registrations and transplants, and deaths, during 3-4 years following
introduction of the legislation. 113 citations.
Hawton K, Bergen H, Simkin S, Dodd S, Pocock P, Bernal W, Gunnell D,
Kapur N (2013). Long term effect of reduced pack sizes of paracetamol on
poisoning deaths and liver transplant activity in England and Wales:
interrupted time series analyses. British Medical Journal (BMJ)
346: f403. doi:10.1136/bmj.f403.
Demonstrated long-term beneficial impact of the 1998 legislation on
liver unit registrations for transplantation and deaths from paracetamol
overdose in the 11 years since the introduction of the 1998 legislation.
4 citations. See Section 4, Details of the Impacts.
Major grants to Hawton supporting the underpinning research
• 1993-8. Department of Health. (£263K). Oxford Monitoring System
for Attempted Suicide.
• 1998 Anglia & Oxford Region R&D NHS Executive (£39,795)
Evaluation of the effects of legislation about the availability of
paracetamol and aspirin on suicide and deliberate self-harm.
• 2001 South-East Region R&D NHS Executive (£66K) `Evaluation of the
longer-term effects of UK analgesic pack legislation on mortality and
morbidity associated with paracetamol and salicylate self poisoning.'
• 2007 National Institute for Health Research (£926K) `A multicentre
programme of clinical and public health research in support of the
National Suicide Prevention Strategy for England.'
Throughout this period, Professor Hawton was Director of the Centre for
Suicide Reseach at Oxford University, and an honorary consultant
psychiatrist. Key Oxford colleagues for the underpinning research included
Joan Fagg, Sue Simkin and John Deeks, plus collaborator Nav Kapur
(Manchester).
Details of the impact
Hawton's research on paracetamol, and the consequent change in
legislation, has had impacts since 2008 in addition to those which
occurred previously.
Sustained reduction in UK deaths from paracetamol poisoning
The detailed analysis of Hawton et al. (2013) shows that 68 fewer deaths
occur in the UK each year from paracetamol poisoning than would have
expected to occur without the change in legislation. This number reflects
the difference between the green and red lines (shown in the Figure,
reproduced from the paper), and equates to a 43% reduction. There is no
evidence that the effect is tailing off over time: the effect persists
and remains significant during the REF2014 impact period. The data
show an estimated 136 lives saved in 2008 and 2009 (and a total of 765
fewer deaths since 1998); extrapolation across the whole REF2014 impact
period (January 2008-June 2013) gives a figure of 374 lives saved.
(Section 5, Source 1).
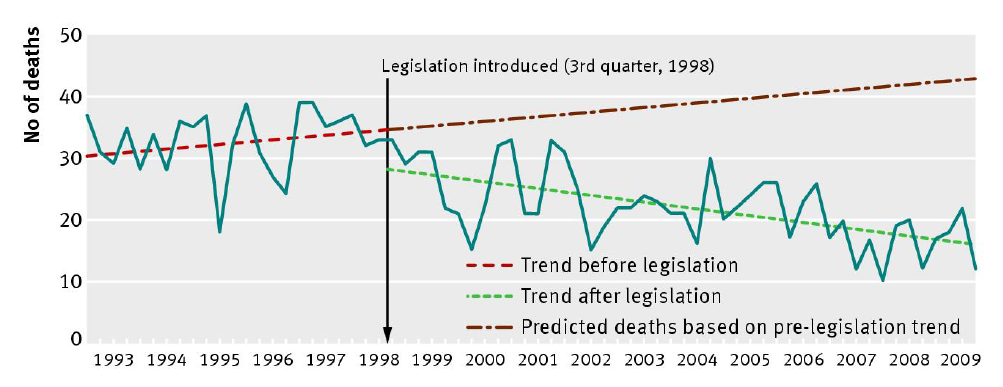
The paper also shows that, even under the most conservative assumptions
(e.g. that paracetamol deaths would have remained constant after 1998
rather than continuing their upward trend, and adjusting for other
potential confounding factors), the legislation has saved 40 lives per
year (and a predicted 220 during the REF2014 period).
The significance of Hawton's work and the change in legislation is
acknowledged by the MHRA and the Chair of the National Suicide Prevention
Strategy (Section 5, Sources 2 and 3).
Hawton et al. (2013) also show a sustained effect on registrations for
liver transplantation due to paracetamol toxicity, with an estimated 61%
reduction, corresponding to 44 fewer registrations per year, and 242
during the REF2014 impact period. However, using the more conservative
analyses mentioned above, the reduction becomes smaller, and no longer
statistically significant.
In a separate study (Simkin et al., Quarterly Journal of Medicine,
2012; 105; 41-51), Hawton's group had shown that at least one retailer was
circumventing the law by offering `multi-buy' deals for paracetamol.
Because the Hawton et al. (2013) analysis showed clearly the continuing
effectiveness of the legislation, the UK Royal Pharmaceutical Society
highlighted the problem and called for further legislation to outlaw the
practice (Section 5, Source 4).
Changes in legislation in other jurisdictions
Since 2008, three other countries have announced a restriction on
paracetamol pack sizes, based on the findings of Hawton's research:
Denmark, The Netherlands, and Australia (Section 5, Sources 6-9). These
are in addition to the countries that had done so before 2008.
Sources to corroborate the impact
Sustained reduction in UK deaths and liver toxicity from paracetamol
poisoning
- Hawton, K., Bergen, H., Simkin, S., Dodd, S., Pocock, P., Bernal, W.,
Gunnell, D. & Kapur, N. (2013). Long term effect of reduced pack
sizes of paracetamol on poisoning deaths and liver transplant activity
in England and Wales: interrupted time series analyses. BMJ, 346, f403.
doi:10.1136/bmj.f403.
- Letter on File: Director, Medicines and Healthcare products Regulatory
Agency (MHRA), 2012. Includes: `The restriction of access to
paracetamol...has been an important regulatory intervention to
protect public health....Your research team's contribution was pivotal
in providing a basis for initiating regulatory change in 1997-8...Your
most recent research, reviewing over 10 years of follow-up, has shown
a significant reduction in deaths from paracetamol overdose of
approximately 38%, and a parallel reduction in registrations for liver
transplantation for paracetamol overdose'.
- Letter on File: Professor Louis Appleby, Chair of National Suicide
Prevention Strategy for England Advisory Group and former Director of
Mental Health for England:
`Research by Prof Keith Hawton on suicide and self-harm has had a
major impact on suicide prevention policy and practice in this
country. His studies of paracetamol self-poisoning led to the current
restriction on over-the-counter sales. He has [shown] a reduction in
fatal and non-fatal paracetamol overdoses and the need for liver
transplantation. The national suicide prevention strategy for England
was revised in line with recent research, and published in 2012. One
of its main aims is to reduce access to certain methods of suicide,
using the evidence on paracetamol as an example...findings from Prof
Hawton's research have been and remain highly influential'.
- UK Royal Pharmaceutical Society, 2013:
`Pharmacists want ban on multi-buy deals on paracetamol: The UK Royal
Pharmaceutical Society [RPS] has called for legislation to stop
retailers offering multi-buy deals on paracetamol, after new research
published by the BMJ [Hawton et al., 2013] showed that limiting
paracetamol pack sizes had cut deaths from overdose of the drug.
Voluntary guidelines exist, but the retailer Poundland contravened
these in 2012...'. The RPS statement was issued on their website
but link is no longer active (available on request). The statement was
published in BMJ 2013;346:f977 doi: 10.1136/bmj.f977 (Published
13 February 2013).
- BBC News, 8th February 2013. `Fall in paracetamol deaths
linked to pack limits.' http://www.bbc.co.uk/news/health-21370910.
Describes results of the Hawton et al (2013) BMJ paper, and includes a
quote from the Samaritans: `its encouraging to see that legislation
can have an effect on reducing suicides'.
Changes in legislation in other jurisdictions
- Denmark: Letter on File, Prof. Nordentoft, Mental Health Centre,
University of Copenhagen:
`...papers from University of Oxford, conducted by Professor Keith
Hawton and his group played an important role in the discussions with
health authorities, and the paper `Long term effect of reduced pack
sizes of paracetamol on poisoning deaths and liver transplant activity
in England and Wales: interrupted time series', BMJ 2013, played a
crucial role in the process of finally recommending pack size
restrictions after the model used in England and Wales.... The Danish
Health Minister of Health has now proposed packet size restrictions to
the Danish Parliament, and the new regulations will be effective from
summer 2013. This would not have happened if it hadn't been for the
tireless work of Professor Hawton and his group'.
- Denmark: Letter on File, Ministry of Health, confirming the above
decision and that it was `based on new data from new international
studies...'. [translated from Danish].
- The Netherlands: Letter on File, Prof. Kerkhof, Free University
Amsterdam:
`In 2008 we advised the [Dutch] Ministry of Health to reduce the
packaging size of paracetamol...we based this advice largely upon your
work...as of 2013 only small packs are available. I consider this as a
big success, and it really is one of the outcomes of your research
into this important suicide prevention strategy'.
- Australian Therapeutic Goods Administration (2013) decision to further
reduce paracetamol pack size (to harmonize with New Zealand).
http://tga.gov.au/newsroom/media-2013-paracetamol-130826.htm As
part of the explanation for the decision, it states `In the UK, the
paracetamol pack sizes have been reduced, and there has been a
corresponding reduction in paracetamol-related deaths, hospital
admissions, liver transplants'.