The development and commercialisation of a polymer that reduces microbial colonisation on dental surfaces, thus improving oral health
Submitting Institution
University of PortsmouthUnit of Assessment
Allied Health Professions, Dentistry, Nursing and PharmacySummary Impact Type
TechnologicalResearch Subject Area(s)
Chemical Sciences: Physical Chemistry (incl. Structural)
Engineering: Biomedical Engineering, Materials Engineering
Summary of the impact
A team of Portsmouth researchers has developed a transparent polymer
coating that prevents colonising bacteria from adhering to the surfaces of
teeth. In addition to protecting from decay, the polymer coating has the
added benefits of reducing dental erosion, alleviating root
hypersensitivity, and inhibiting the staining of teeth. GlaxoSmithKline
(GSK) has adopted this technology and the polymer has been successfully
developed into a component of "next-generation" oral healthcare products.
Underpinning research
The nature of the research insights or findings
Interactions between solid surfaces and microorganisms are of importance
in areas such as biomaterials, biotechnology, clean-room techniques or the
coatings industry. For example, materials for the biomedical device market
(implants, valves, grafts, pacemakers, bone repair and replacement
devices, artificial organs, dental materials, drug-delivery systems,
separation systems, catheters and stents) accounts for annual sales of
several billion US dollars. In another example, the protection of ships
and submerged structures from marine biofouling is a major industry.
Portsmouth researchers were the first to develop the "ultra-low surface
energy" approach to the inhibition of bacterial colonization.1,2
Outline of what the underpinning research produced
The hypothesis underpinning the approach is based on the well-established
Derjaguin-Landau-Verwey-Overbeek (DLVO) theory for the stability of
colloidal dispersions: since in natural aqueous environments most surfaces
and most bacteria (colloidal dispersions) are repelled by their respective
negative charges, the utilisation of "ultra-low surface energy" coatings
would minimize the competitive attractive (van der Waals) interactions
operating between the target surface and the prospective coloniser,
preventing the attachment of the bacterium onto that surface.
Key to the testing of the approach has been the molecular design3
of fluoropolymers with time-independent surface energies of ca 6
mJ m-2 (the archetypal non-stick material
polytetrafluoroethylene (PTFE, Teflon; surface energy ca. 18 mJ m-2)
is highly susceptible to colonisation by bacteria), and the development of
pharmaceutically relevant methods for coating biological (teeth4,5)
and other (glass, plastic, metal6) substrates. To this end,
methods have been developed for the radical-induced, chain-growth
synthesis of fluoropolymers in bulk, in solution, in suspension and, more
importantly, in aqueous emulsion such that the fluoropolymers may be
amenable to formulation in toothpastes, sprays, mouthwashes, gels,
lozenges, chewing gums, tablets, pastilles, powders, oral strips and
buccal patches.
Tested against a selection of bacteria and other fouling organisms,
coated structures exhibited resistance to colonisation, validating the
approach (see Figure 1).1 In vivo (rat model)
experiments, performed with reference to potential biomedical
applications, confirmed the biocompatibility of the materials.1
(a)
(b)
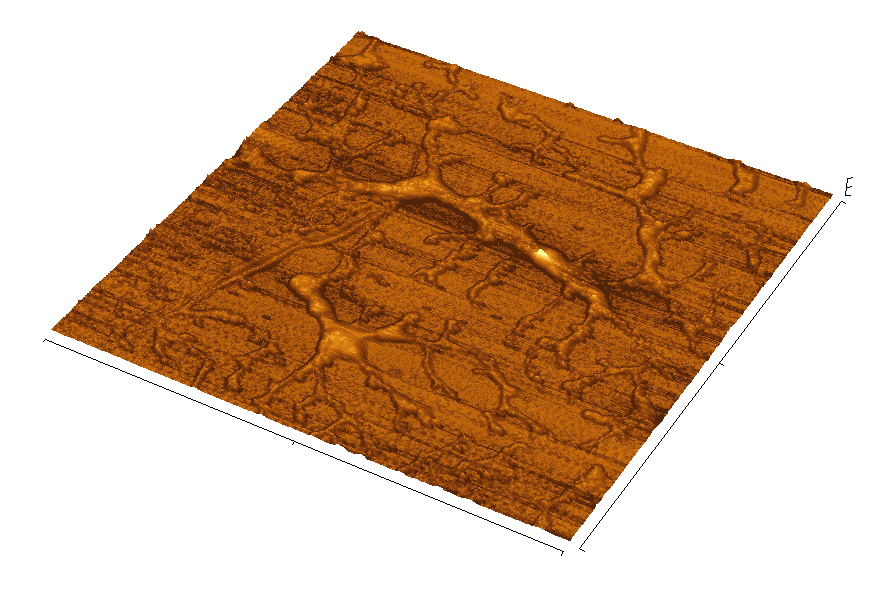
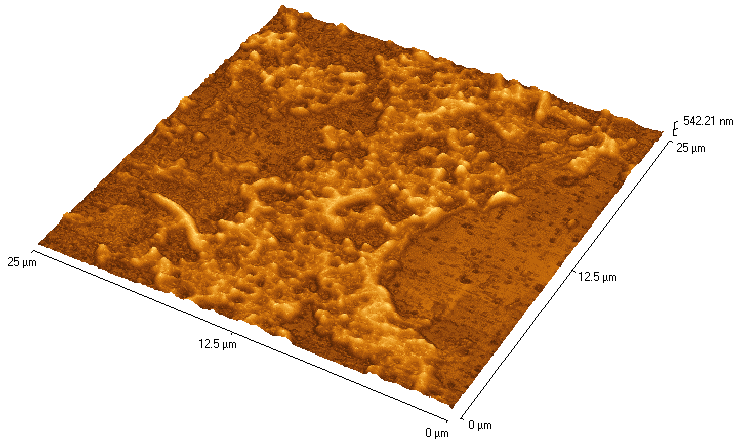 Figure 1. The Portsmouth fluoropolymer at work
Figure 1. The Portsmouth fluoropolymer at work
Atomic Force Microscope (AFM) images of coated surfaces are shown after a
two-week exposure to a bacterial culture (Desulfovibrio alaskensis).
In (a), the surface had been pre-treated with the fluoropolymer,
poly (fluoroalkylacrylate). Bacterial colonies were seen to slide off this
ultra-low energy surface as it was raised out of the culture broth. The
surface in (b) had been pre-treated with a control polymer (PMMA).
Bacterial colonies attached to this control surface could only be removed
by physical abrasion.
Dates, names of key researchers and contextual information
This research project was initiated by Drs John Tsibouklis (Senior
Lecturer 1994-1999; Reader 1999) and Thomas Nevell (Principal Lecturer;
retired 2006) and received financial support from EPSRC and NERC (1994 - 1997,
see also below). Following the publication of early scientific
findings, industrial organisations, including GlaxoSmithKline (GSK) Dental
Health, became interested in the exploitation of aspects of the
technology.
References to the research
The underpinning research described above has been published in some of
the top international journals in biomaterials and pharmaceutics. Funding
for the research has been provided by UK research councils (BBSRC, EPSRC,
NERC) and leading global pharmaceutical / healthcare industries (GSK and
its predecessor, SKB).
1. Tsibouklis J, Stone M, Thorpe AA, Graham P, Peters V, Heerlien
R, Smith JR, Green KL, Nevell TG (1999). Preventing bacterial adhesion
onto surfaces: the low-surface-energy approach. Biomaterials,
20(13), 1229-123. DOI: 10.1016/S0142-9612(99)00023-X
2. Tsibouklis J, Graham P, Eaton PJ, Smith JR, Nevell TG, Smart
JD, Ewen RJ. (2000) Poly (perfluoroalkylmethacrylate) Film Structures:
Surface Organisation Phenomena, Surface Energy Determinations and Force
of Adhesion Measurements. Macromolecules, 33(22),
8460-8465. DOI: 10.1016/S0032-3861(01)00777-7
3. Tsibouklis J, Nevell TG (2003) Ultra-low surface energy
polymers: the molecular design requirements. Advanced Materials,
15(7-8), 647-650. DOI: 10.1002/adma.20030168
PDF copy can be supplied by the institution on request.
4. Churchley D, Rees GD, Barbu E, Nevell TG, Tsibouklis J (2008)
Fluoropolymers as low-surface-energy tooth coatings for oral care.
International Journal of Pharmaceutics, 2008, 352, 44-49. DOI:
10.1016/j.ijpharm.2007.10.024 Ref2 output: 3-JT-004
5. Nielsen BV, Nevell TG, Barbu E, Smith JR, Rees GD, Tsibouklis J,Multifunctional
poly(alkyl methacrylates)s films for dental care. Biomedical
Materials 2011, 6, 015003. DOI: 10.1088/1748-6041/6/1/015003
6. Mark A. McHugh, Alberto Garach-Domech, Il-Hyun Park, Paul Graham,
Eugen Barbu, John Tsibouklis (2002), Impact of fluorination and
side-chain length on poly(methylpropenoxyalkyl siloxane) and poly(alkyl
methacrylate) solubility in supercritical carbon dioxide Macromolecules,
35(17), 6479-6482. DOI: 10.1021/ma012169i
External research funding to J. Tsibouklis and co-workers / UoP
BBSRC/SmithKlineBeecham. "Adherent oral coatings"; £46,000 awarded
to J. Smart and J. Tsibouklis; 1998-2001.
EPSRC/GlaxoSmithKline. "Polymers for oral care products"; £58,500
awarded to J. Smart and J. Tsibouklis; 2001-2004.
BBSRC/GlaxoSmithKline. "Antimicrobial/polymer complexes"; £60,000
awarded to J. Tsibouklis; 2002-2005.
GlaxoSmithKline. "Multifunctional polymers for dental care",
£70,450 awarded to J. Tsibouklis; 2004-2007.
GlaxoSmithKline. "Multifunctional films for dental care", £106,000
awarded to J. Tsibouklis; 2004-2007.
Details of the impact
In order to establish a colony on a surface, a microbe must first be able
to adhere to that surface. Preventing such adherence is therefore a
logical strategy for the prevention of microbial colonisation to the
surfaces of biomedical implants, artificial organs and dental enamel, to
name but a few. Microbial adhesion to teeth is a prerequisite to decay, so
treatments that inhibit adhesion are prime candidates for inclusion in
oral hygiene products. Furthermore, such dental coatings not only have the
potential to reduce caries (cavity) formation, but also to limit the
acid-induced demineralisation of enamel, the increased permeability of
dentine (which leads to dental hypersensitivity), and the unsightly
staining of tooth surfaces by, for example, spices, tannins and nicotine.
The Portsmouth team's innovative approach of using their ultra-low
surface energy fluoropolymers to reduce bacterial colonization received
considerable media attention (ref. 1). In turn, this triggered the
attention of several industrial partners, each of whom signed evaluation
licenses in the technology. These partners' application interests ranged
from the inhibition of marine biofouling (DowCorning), through the
prevention of soiling in engineering tools (Unilever), to uses in domestic
household products (Reckitt Benckiser) and biomedical materials
(GSK). However, the greatest impact from the Portsmouth Team's research
has been in the arena of oral healthcare through an ongoing
collaboration with GSK (ref. 2).
During the development of their fluoropolymer as a dental care
material, the Portsmouth team, in collaboration with GSK, found that (1)
the fluoropolymer coating, when deposited from aqueous emulsion,
greatly reduces demineralisation of dental hard tissues by dietary
acids, and the consequential tooth wear and erosion; (2) the fluoropolymer
reduces dentine permeability as evaluated by the industry-standard
hydraulic conductance model; (3) inhibits the adhesion to dental
substrates of primary oral colonisers (S. sanguinis, A. naeslundii),
a major aetiological pathogen implicated in dental caries (S. mutans),
and a mixed bacterial culture isolated from human saliva; and (4) inhibits
extrinsic staining by tooth chromogens such as the polyphenolic components
of tea, coffee and red wine.
The obvious commercial potential of the Portsmouth team's fluoropolymer
was immediately recognized by GSK who subsequently filed for patent
protection of the intellectual property relating to the design of the
fluoropolymer itself, to a process for its preparation, to oral care
compositions comprising such polymers, and to its use in the prevention,
inhibition and treatment of dental erosion, tooth wear, dentine
hypersensitivity, anti-staining of dental enamel and anti-adhesion of oral
bacteria. (ref. 3)
When added to toothpaste and/or mouthwash, such materials provide an
efficient, biocompatible and cost-effective means of limiting the damage
to teeth by oral bacteria. Therefore, the development of these innovative
compounds as inhibitors of bacterial adhesion to dental surfaces has wide
ranging benefits in enhancing the oral health of the general public,
not only in the UK, but also worldwide. In the words of the Director of
Gum Health and Dry Mouth Research and Development at GSK (ref.
2), collaborations with the Portsmouth team "have been very
successful and have resulted in next generation products which have a
big impact on the quality of life of patients and consumers." GSK
toothpastes are sold world-wide and include many well-known brands in the
UK, such as Sensodyne® and Aquafresh®.
Sources to corroborate the impact
1. Examples of the press coverage of the discovery of the ultra
low-surface-energy approach as a non-toxic means for the inhibition of
surface colonisation by bacteria;
1. The Guardian, 25/3/1999
http://www.theguardian.com/technology/1999/mar/25/onlinesupplement1
2. BBC Radio 4 (29/4/1999, 4.30 pm)
2. Letter
A letter from GSK acknowledging the contribution of the University of
Portsmouth team to the development of next-generation oral healthcare
products.
3. Patent
WO2007141330-A1, 13 Dec 2007. To: Eugen Barbu, David Churchley,
Thomas G.Nevell, Gareth D. Rees and John Tsibouklis — assigned to GSK.
http://www.google.com/patents/WO2007141330A1
Patent that protects the invention that relates to a novel polymer, to
a process for its preparation, to oral care compositions comprising such
polymer and to its use in the prevention, inhibition and treatment of
dental erosion, tooth wear, dentine hypersensitivity, anti-staining of
dental enamel and anti-adhesion of oral bacteria