Sentinel lymph node biopsy in breast cancer
Submitting Institution
Queen Mary, University of LondonUnit of Assessment
Clinical MedicineSummary Impact Type
HealthResearch Subject Area(s)
Medical and Health Sciences: Clinical Sciences, Oncology and Carcinogenesis
Summary of the impact
In breast cancer surgery the major physical side effects occur as a
result of surgery to the axilla. Research at Queen Mary showed
significantly reduced rates of axillary clearance surgery and physical
side effects, and significantly improved quality of life, in breast cancer
patients managed with sentinel lymph node biopsy (SLNB) compared with
patients undergoing axillary lymph node dissection. As a result, SLNB was
recommended in NICE guidance and is now routine in most breast centres.
Previously, only 10% of UK breast surgeons used the procedure, but by 2010
this had increased to 64%. We estimate around 3,500 patients are spared
lympheodema in the UK every year as a result of this research.
Underpinning research
Because breast tumours most commonly spread first via the lymph nodes in
the axilla, removing and checking these for cancer cells is essential for
staging the tumour and making consequent adjuvant therapeutic decisions.
Removal of tumour-positive lymph nodes may also have a therapeutic
benefit, but this is not fully established. Axillary lymph node dissection
(ALND) is associated with significant morbidity, including loss of arm
mobility, loss of feeling and lymphoedema (arm swelling), which can be
prolonged, distressing and disabling. Lymphoedema may lead to ulceration
or infection if severe.
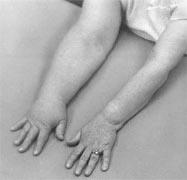 Figure 1: Lymphoedema of the right arm following axillary lymph node
dissection
Figure 1: Lymphoedema of the right arm following axillary lymph node
dissection
The principle of SLNB is that if the first two or three (sentinel) lymph
nodes to which the tumour drains are located and have no tumour cells
present, further surgery to the axilla can be dispensed with, potentially
minimizing or avoiding these serious side effects. Identification is done
by injecting a dye, radioisotope or both in the affected breast and
identifying the nodes to which the marker substance drains. The aim of
this research was to characterise and quantify physical and psychological
morbidity after SLNB in the treatment of early breast cancer in a
randomized controlled trial.
Between November 1999 and February 2003, 298 patients with early breast
cancer (tumours measuring 3cm or less on ultrasound examination) who were
clinically node-negative were recruited from three hospitals in England,
and randomly allocated to undergo ALND (control group) or SLNB followed by
ALND if subsequently found to be lymph node positive (study group). A
detailed assessment of physical and psychological morbidity was performed
on trial participants during a one-year period postoperatively.
The main findings from this trial were:
- Significant reduction in postoperative arm swelling, rate of seroma
formation, numbness, and loss of sensitivity to light touch and pinprick
in the intervention group compared with controls.
- Quality of life and psychological morbidity scores were significantly
better in the intervention group in the immediate postoperative period,
with fewer long-term differences.
- The odds of arm lymphoedema were reduced by 75% at six months in the
study group as a whole, and by 82% in those who were node negative. At
six months, 21% of participants receiving axillary clearance had
lymphoedema compared with 7% in those receiving sentinel lymph node
biopsy.
- Incidence of seroma formation was reduced from 21% in the control
group to 14% in the intervention group. In the node-negative cases, the
difference was greater, at 24% vs 11%. (The effect is greatest in the
node-negative cases, as these are the cases who do not go on to further
axillary surgery.)
This trial, published in the Journal of Clinical Oncology in
2005, concluded that SLNB in patients undergoing surgery for breast cancer
results in a statistically and clinically significant reduction in
physical and psychological morbidity [1]. Downstream research within the
same study identified factors predictive of further tumour cells in the
axilla in sentinel node positive patients [2]; and showed that in those
undergoing axillary clearance surgery, those at most risk of arm
lymphoedema were the node-negative patients [3]. This is of particular
importance as it is the node-negative patients who will have axillary
clearance avoided as a result of sentinel node biopsy.
Stephen Duffy, Professor of Cancer Screening at QMUL, was a co-applicant
on the trial grant, designed the trial, carried out the randomisation and
supervised the statistical analysis [1]. A research associate at QMUL, JP
Myles, carried out the primary statistical analysis under Duffy's
supervision [1]. A research associate at QMUL, OF Agbaje, and honorary
research associate at QMUL, P Chou, carried out secondary analyses under
Duffy's supervision [2, 3].
References to the research
1. Purushotham AD, Upponi S, Klevesath MB, Bobrow L, Millar K, Myles
JP, Duffy SW. Morbidity after sentinel lymph node biopsy in primary
breast cancer: Results from a randomized controlled trial. Journal of
Clinical Oncology 2005; 23: 4312-21.
2. Pal A, Provenzano E, Duffy SW, Pinder SE, Purushotham AD. A
model for predicting non- sentinel lymph node metastatic disease when the
sentinel lymph node is positive. British Journal of Surgery 2008;
95: 302-9.
3. Purushotham AD, Bennet Britton TM, Klevesath MB, Chou P,
Agbaje O, Duffy SW. Lymph node status & breast cancer-related
lymphoedema. Annals of Surgery 2007; 246: 42-5.
The trial was funded by the Eastern Region R&D Directorate and
supported by the National Cancer Research Network. Grant holders were
Purushotham et al, the title was `Sentinel lymphadenectomy in
breast cancer: a randomised trial', the sponsor was Addenbrookes Hospital,
Cambridge. The grant ran from 1999 to 2003 and totalled £175,000.
Details of the impact
The study findings led directly to a change in guidelines, a change in
clinical practice and quantifiable improvements in patient morbidity.
4a: Change to national and international recommendations
Four examples of clinical guidelines around the world:
-
UK: The SLNB technique is now recommended by the UK National
Institute for Health and Clinical Excellence (NICE) [4]: "Sentinel
node biopsy ((SLNB) or axillary four node sampling) should be
performed to stage the axilla for patients with early invasive breast
cancer and no evidence of lymph node involvement on ultrasound or a
negative ultrasound-guided needle biopsy. SLNB is the preferred
technique."
-
UK: Surgical guidelines in the UK were updated to include the
recommendation for sentinel node biopsy following the study publication
in 2005 [5]. "To minimise morbidity from axillary surgery to obtain
staging information Sentinel node biopsy using the combined blue
dye/radioisotope technique is a recommended axillary staging procedure
for the majority of patients with early invasive breast cancer".
-
Europe: The European Society for Medical Oncology
recommend sentinel node biopsy as the standard of care in early breast
cancer [6].
-
USA: SLNB is endorsed as an alternative to ALND for the
diagnosis of axillary metastases in patients with clinically
node-negative early breast cancer in guidelines from the American
Society of Clinical Oncology (ASCO), which cites this trial as evidence
for the reduction in surgery-related morbidity [7], and by the US
National Comprehensive Cancer Network Guidelines [8].
-
Australia: The Australian guidelines on management of breast
cancer recommend SLNB and explicitly cite this trial as evidence of the
reduction in side effects [9].
4b: Change to standard clinical practice
SLNB has become routine in most breast centres. A survey published in
2010 reported that 69% of breast surgeons in the UK routinely use the
practice now [10], compared with 10% before this trial was published [11].
Increasing trends in the use of SLNB have also been reported from Canada
and Ireland [12,13].
4c: Development of new surgical training
Following the trial, successful training programmes have been
established, aimed primarily at surgical technique, but also incorporating
multidisciplinary training for nuclear medicine physicians, theatre nurses
and pathologists [14].
4d: Reduction in morbidity
Around 38,000 women are diagnosed with invasive breast cancer in England
per year. The Second All Breast Cancer Report reported 32% of patients
receiving sentinel node biopsy in 2007 [15]. However, more than half the
patients had missing data on axillary surgery in this audit. The audit of
screen-detected cancers for 2010/11 reported that 76% of screen-detected
cancers had sentinel node biopsy [16]. A local audit in Staffordshire
reported 84% of patients with screen- detected cancers receiving sentinel
node biopsy [17]. Taking the average of these three figures (probably
conservative because the proportion is increasing with time), an estimated
64% of eligible UK patients currently have this procedure. This estimate
is consistent with a survey of UK surgeons in 2008, which found that 69%
routinely performed the operation [10].
The low incidence of lymphoedema observed in cohorts of patients treated
with SLNB [18] compared with observations in the pre-SLNB era (see for
example British Journal of Surgery 2000; 87: 1128) is consistent
with the numbers reported in our study. This translates to 3,405 patients
per year in UK (0.64 x (0.21-0.07) x 38,000) spared lymphoedema due to
this procedure.
4e Extension of the technique to other cancers
The first cancer for which SLNB was introduced was melanoma. Following
the success of this approach in breast cancer, researchers have begun to
explore the use of the technique in other cancers, most notably of the
head and neck [19].
Sources to corroborate the impact
- NICE Guideline 2009 `Breast cancer (early and locally advanced):
diagnosis and treatment
http://www.nice.org.uk/CG80
(reviewed 2012 and confirmed still current).
- Association for cancer surgery (BASO): Surgical Guidelines for the
Management of Breast Cancer. European Journal of Surgical Oncology
2009, S1-S22. doi:10.1016/j.ejso.2009.01.008
http://www.associationofbreastsurgery.org.uk/media/4565/surgical_guidelines_for_the_management_of_breast_cancer.pdf
- Aebi S, Davidson T, Gruber G, et al. Primary breast cancer:
ESMO clinical practice guidelines for diagnosis, treatment and follow-up.
Annals of Oncology 2011; 22: S6 vi12-vi24.
http://www.esmo.org/education-research/esmo-clinical-practice-guidelines/topics/breast-cancer.html
- Lyman GH, Giuliano AE, Somerfield MR, et al. American Society
of Clinical Oncology guideline recommendations for sentinel lymph node
biopsy in early-stage breast cancer. Journal of Clinical Oncology
2005; 23: 7703-20.
http://jco.ascopubs.org/content/23/30/7703.long
- Australian national breast cancer guideline:
http://guidelines.nbocc.org.au/guidelines/sentinel_node_biopsy/
- Glynn RW, Williams L, Dixon JM. A further survey of surgical
management of the axilla in UK breast cancer patients. Annals of the
Royal College of Surgeons of England 2010; 92: 506-11.
- Gaston MS,Dixon JM. A survey of surgical management of the axilla in
UK breast cancer patients. European Journal of Cancer 2004; 40:
1738-42.
- Quan ML, Hodgson N, Lovrics P et al. National adoption of
sentinel node biopsy for breast cancer: lessons learned from the Canadian
experience. The Breast Journal 2008; 14:421-7.
- Heneghan HM, Prichard RS, Devaney A, et al. Evolution of
breast cancer management in Ireland: a decade of change. BMC Surgery
2009; 9: 15.
- Somasundaram SK, Chicken DW, Keshtgar MRS. Detection of the sentinel
lymph node in breast cancer. British Medical Bulletin 2007; 84:
117-31. (outline training curriculum)
- NHS Cancer Screening Programme and National Cancer Intelligence
Network. The Second All Breast Cancer Report, 2011. http://www.ncin.org.uk/view?rid=612
- NHS Breast Screening Programme and Association of Breast Surgery. An
Audit of screen-detected breast cancers for the year of screening April
2010 to March 2011 (see page 12).
http://www.cancerscreening.nhs.uk/breastscreen/publications/baso2010-2011.pdf
- Apostolopoulos A, Basit A, Kirby RM et al. Conservation of
the axilla: an audit of sentinel lymph node biopsy after a new start. Clinical
Breast Cancer 2011; 11: 264-7.
- McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of
Lymphedema in Women With Breast Cancer 5 Years After Sentinel Lymph Node
Biopsy or Axillary Dissection: Objective Measurements. Journal of
Clinical Oncology 2008; 26: 5213-9.
- Rigual N, Loree T, Frustino J et al. Sentinel node biopsy in
lieu of neck dissection for staging oral cancer. JAMA Otolaryngology and
Head Neck Surgery 2013; 139: 779-782.