Treatment of adult growth hormone deficiency
Submitting Institution
Queen Mary, University of LondonUnit of Assessment
Clinical MedicineSummary Impact Type
HealthResearch Subject Area(s)
Medical and Health Sciences: Clinical Sciences
Summary of the impact
Research at Queen Mary established the beneficial effects of adult growth
hormone (GH) replacement.
Prof Korbonits' team pioneered careful GH dose titration and adjustment of
other concomitant pituitary
hormone replacements, both crucial for effective and safe treatment.
Collaborative research between
adult and paediatric endocrinologists established a new model of GH
deficiency treatment between
completion of linear growth and full maturity at age 25. Findings led to
[a] revised guidelines and policy
in UK, Europe and USA; [b] new service models (especially at the
paediatric-adult transition); [c]
significant changes in clinical practice, [d] improved patient outcomes,
notably dramatically improved
quality of life, reduced cardiovascular risk and improved survival, [e]
reduced costs.
Underpinning research
Background: Hypopituitarism affects at least 1 in 10,000 people.
The commonest cause is pituitary
tumours. The observation in 1990 of increased mortality in adult
hypopituitary patients on conventional
hormone replacement regimens (standardised mortality ratio, SMR,
approximately 2:1 and 3:1 in males
and females respectively) prompted detailed examination of the metabolic
consequences of hormonal
deficiencies, especially the impact of GH deficiency and replacement.
Aim: To evaluate the impact of GH replacement in adult and adolescence
hypopituitary patients,
optimise dose regimens, document changes in cardiovascular and bone risk
factors and quality
of life, and explore how best to adjust other pituitary hormone
replacement.
Key studies and findings: The Department of Endocrinology at Queen
Mary, led by Professor John
Monson and including Profs Drake & Gelding, Drs Carroll, Swords, Agha,
Brooke and Weaver, was at
the forefront of this endeavour in collaboration with paediatric
endocrinologist Prof Martin Savage and
team. From 1993, using prospective placebo-controlled and observational
studies, the team has been
made pivotal contributions to the knowledge base on GH deficiency.
Highlights include:
- The team demonstrated that GH deficient patients have a highly adverse
cardiovascular risk
profile and that this risk could be significantly reduced with GH
replacement. In particular, we
have shown systematically that GH replacement improves cardiovascular
risk factors e.g. it
reduces LDL-cholesterol by a mean of 0.4mmol/l [1].
- This department was the largest contributor to the major multinational
database (KIMS)
examining the impact of GH replacement on various indices of well-being
and health and these
data led to the introduction of the 2003 NICE guidelines on adult GH
deficiency. This database
has been crucial in documenting the impact of GH replacement on
morbidity and mortality in a
large cohort of GH deficient patients (see `Impact' below).
- The research showed that GH replacement significantly improves overall
well-being and
energy levels, as demonstrated by reduction of the disease-sensitive
QoL-AGHDA (Quality of
Life — Assessment of GH Deficiency in Adults) score from median 15 to 7
[2,3].
- The team tested a number of approaches to establish the appropriate
method of careful dose-optimisation
for GH replacement in adults and adolescents [4].
- The team established optimal management of GH deficiency in the
critical period between
adolescence and adulthood, when linear growth is complete, which had
previously been the
subject of much clinical debate [4,5,6,7]. A structured programme of
collaborative clinical care
led the way in establishing a model for optimal paediatric to adult
transitional care of endocrine
disorders. The model, sited in the adult endocrine clinic, staffed by
paediatric and adult
endocrinologists and specialist nurses, was the first of its kind in the
UK. Subsequent close
paediatric-adult research collaboration led to original data that was
pivotal in the 2003 NICE
Guidance on GH therapy in GH deficient patients during transition from
paediatric to adult care.
- In addition to the observed bone density improvement in adults [5], in
adolescents the team
showed, in a multicentre controlled trial co-ordinated from Queen Mary,
that GH treatment after
completion of linear growth until acquisition of peak bone mass improves
bone mineral density
by 4% and lean body mass by 4% over 1 year, compared to non-treated
controls [6,7].
- In prospective interventional studies they have demonstrated the
important interactions
between GH replacement and other hormonal systems, particularly the
thyroid axis, and the
effect of GH on increasing metabolic clearance of cortisol and the
potential adverse effect of
this phenomenon in patients with partial ACTH deficiency [8,9].
- They have shown a reduction on societal and healthcare costs for GH
deficient patients [3].
- The adverse impact of GH deficiency on psychological well-being and
energy levels, and the
benefits of GH replacement on these aspects were clearly demonstrated.
This provided the major
evidence base for NICE guidance on the treatment of GH deficiency (see
`Impact').
References to the research
1. Weaver JU, Monson JP, Noonan K, John WG, Edwards A, Evans KA,
&Cunningham J. The
effect of low dose recombinant human growth hormone replacement on
regional fat
distribution, insulin sensitivity, and cardiovascular risk factors in
hypopituitary adults. Journal of
Clinical Endocrinology and Metabolism 1995; 80: 153-159.
2. Koltowska-Haggstrom M, Mattsson AF, Monson JP, Kind P, Badia X,
Casanueva FF, Busschbach
J, Koppeschaar H P & Johannsson G. Does long-term GH replacement
therapy in hypopituitary
adults with GH deficiency normalise quality of life? European Journal
of Endocrinology 2006; 155:
109-119.
3. Hernberg-Stahl E, Luger A, Abs R, Bengtsson BA, Feldt-Rasmussen U,
Wilton P, Westberg B,
Monson JP. Healthcare consumption decreases in parallel with improvements
in quality of life
during GH replacement in hypopituitary adults with GH deficiency. Journal
of Clinical Endocrinology
and Metabolism 2001; 86: 5277-5281.
4. Drake WM, Coyte D, Camacho-Hübner C, Jivanji NM, Kaltsas G, Wood DF,
Trainer PJ, Grossman
AB, Besser GM, Monson JP. Optimising growth hormone replacement therapy by
dose titration in
hypopituitary adults. Journal of Clinical Endocrinology and Metabolism
1998; 83: 3913-3919. and
Drake WM, Howell SJ, Monson JP, Shalet SM. Optimizing GH therapy in adults
and children.
Endocrine Reviews 2001; 22: 425-450.
5. Drake WM, Rodriguez-Arnao J, Weaver JU, James I, Coyte D, Spector T,
Besser GM, Monson JP.
The influence of gender on the short and long term effects of growth
hormone replacement on bone
metabolism and bone mineral density in hypopituitary adults — a five year
study. Clinical
Endocrinology 2001; 54: 525-532.
6. Carroll PV, Drake WM, Maher KT, Metcalfe K, Shaw NJ, Dunger DB,
Cheetham TD, Camacho-Hubner
C, Savage MO, Monson JP. Comparison of continuation or cessation of growth
hormone
(GH) therapy on body composition and metabolic status in adolescents with
severe GH deficiency
at completion of linear growth. Journal of Clinical Endocrinology and
Metabolism 2004; 89: 3890-3895.
7. Drake WM, Carroll PV, Maher KT, Metcalfe KA, Camacho-Hubner C, Shaw
NJ, Dunger DB,
Cheetham TD, Savage MO, Monson JP. The effect of cessation of growth
hormone (GH) therapy
on bone mineral accretion in GH-deficient adolescents at the completion of
linear growth Journal of
Clinical Endocrinology and Metabolism 2003; 88: 1658-1663
8. Agha A, Walker D, Perry L, Drake WM, Chew SL, Jenkins PJ, Grossman AB,
Monson JP.
Unmasking of central hypothyroidism following growth hormone replacement
in adult hypopituitary
patients. Clinical Endocrinology 2007; 66: 72-77.
9. Gelding SV, Taylor NF, Wood PJ, Noonan K, Weaver JU, Wood DF, Monson
JP. The effect of
growth hormone replacement therapy on cortisol-cortisone interconversion
in hypopituitary
adults: evidence for growth hormone modulation of extrarenal 11
beta-hydroxysteroid
dehydrogenase activity. Clinical Endocrinology 1998; 48: 153-162.
The research was supported by the Wellcome Trust, Queen Mary, NHS and
visiting Fellowship from
Beaumont Hospital and by unrestricted educational pharmaceutical industry
grants.
Details of the impact
4a: Paradigm shift in how GH deficiency is conceptualised
Prior to mid-1990s, GH deficiency was primarily a paediatric disease
focusing on achieving
adequate stature. This research, together with other groups and
collaborators, demonstrated
symptoms and complications of adult GH deficiency, particularly its effect
on lipid metabolism and
(hence) cardiovascular risk, bone and quality-of-life and shown the
importance of replacement after
the cessation of linear growth. Professors Monson and Savage have given
over 30 plenary
lectures and talks on this topic at scientific, clinical and patient care
fora 1993-2013, including
lectures to GPs, trainees, meetings organised by the Royal College of
Physicians and CPD.
4b: Change in national policy and clinical management guidelines
The team's work in adult and transitional-age GH-deficient patients was
critical in informing the
NICE guidance on the management of GHD in adults and the optimum treatment
of this condition
in the transition from childhood to adulthood. The NICE guidance was based
on detailed cost
effectiveness modelling [10]. The work has informed other national and
international clinical
guidelines. In particular, our recommendations feature in the 2002 Society
for Endocrinology
guideline on management of growth hormone deficiency in adults [11].
The Growth Hormone Research Society published a critical evaluation of
the safety of recombinant
HGH administration, which drew on the work of this group [12]. The
team's research into thyroxine
and glucocorticoid replacement established that patients must be
reassessed after initiation of GH
treatment. This recommendation is now also incorporated in drug
prescribing information both in
UK and internationally (see for example this from USA [13]). The new
clinical care model, informed
by this research, of replacing GH in adolescents so as to optimise peak
bone mass and lean body
mass, has now become the standard of care for this patient group
nationally and internationally as
accepted by the European Society of Paediatric Endocrinology [14].
4c: Establishing a new `gold standard' service model
The formal paediatric-adult endocrine service at Barts led the way in
establishing the model for
transitional care of complex endocrine disorders, which is consistent with
the 2006 Department of
Health Guidance publication [15]. This model requires considerably
greater collaboration between
paediatric and adult endocrinology clinics than was previously the case.
4d: Change in clinical practice
Following the team's research publications (mostly between 1995 and
2001), and the incorporation
of their recommendations into NICE guidelines in 2003, prescription rates
for GH in the UK
increased, as confirmed by Department of Health data (Figure 1) [16].
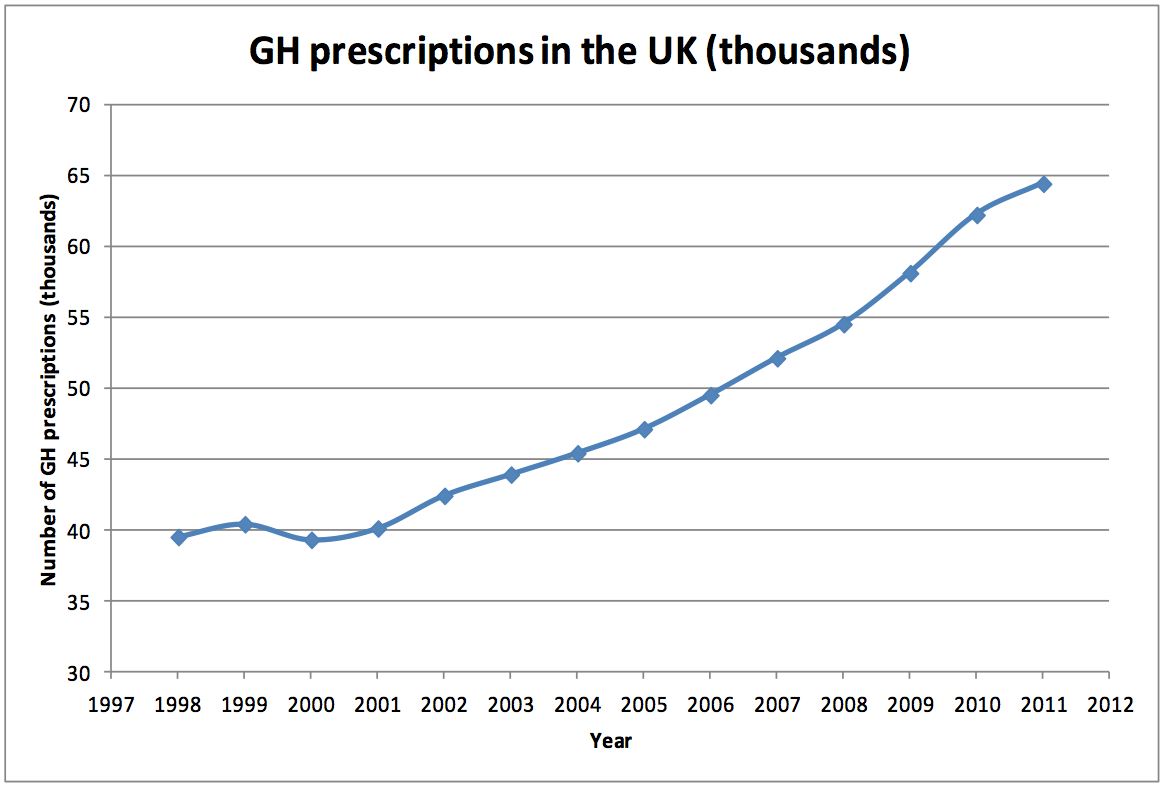 Figure 1; Number of GH prescriptions in the UK by year. The inflection in the curve
starting in 2002 reflects the impact of clinical research in hypopituitary adults.
Figure 1; Number of GH prescriptions in the UK by year. The inflection in the curve
starting in 2002 reflects the impact of clinical research in hypopituitary adults.
4e: Improved clinical outcomes (reduced morbidity)
GH replacement improves health, psychological well-being and quality of
life (reference 3 above).
Cardiovascular risk is reduced (reference 1 above) and real-time data from
the KIMS database
have confirmed that standardised mortality ratios in hypopituitary adults
receiving GH replacement
are lower than the high rates documented in 1990 (2.0 in men and 3.0 in
women) to a mean of 1.2
(0.94 in men and 1.56 in women). KIMS data also demonstrate that these
improvements translate
into a reduction in socio-economic costs (reference 3 above).
4f: Establishing a methodological approach to endocrine research in UK
This team's initiation and leadership of a combined adult-paediatric
endocrinology UK multicentre
study focused on the continuation of GH replacement in adolescents in
optimising peak bone mass
and lean body mass development was the first multi-centre controlled trial
of its kind in this
condition. Since that trial, other high-quality multi-centre trials of
similar design have been initiated
and supported by the clinical endocrinology community — see for example
[17].
4g: Supporting informed choice by patients and informing the public
A summary of these research findings is distributed by the Pituitary
Foundation in a patient booklet
available to download free from their website: `The Pituitary Gland' in
2012 [18]. Direct patient
comments support the positive effects documented in clinical trials [19].
Sources to corroborate the impact
- NICE Guideline. Growth Hormone Deficiency (Adults) — Human Growth
Hormone. TA64
(2003).
http://guidance.nice.org.uk/TA64. Professor Monson is referenced in
Appendix B.
- Society for Endocrinology Guideline on Management of Growth Hormone
Deficiency in Adults
2002. See pages 28 - 33 for references to 10 papers co-authored by Prof
Monson.
http://jcem.endojournals.org/content/86/5/1868.full
- Christiansen JS, Bengtsson BA, Thorner M et al. Critical evaluation of
the safety of
recombinant human growth hormone administration: statement from the Growth
Hormone
Research Society. Journal of Clinical Endocrinology and Metabolism 2001;
86: 1868-70.
http://jcem.endojournals.org/content/86/5/1868.full
- US Food and Drug Administration safety information: Somatropin [rDNA
origin] for injection
2012. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm203732.htm
- European Society for Paediatric Endocrinology recommendation of
transitional care:
http://www.eurospe.org/clinical/Docs/ManagementGH-treatedAdolescentTransitionAdultCare.pdf
- Department of Health guidance on transitional care in complex endocrine
disorders 2006 (still
current).
- Department of Health Prescription Cost Analysis publications 2012:
http://webarchive.nationalarchives.gov.uk/20120104142136/http://www.dh.gov.uk/en/Publicationsandstatistics/Statistics/StatisticalWorkAreas/Statisticalhealthcare/DH_4086488
- Conway GS, Szarras-Czapnik M, Racz K et al. Treatment for 24 months with
recombinant
human GH has a beneficial effect on bone mineral density in young adults
with childhood-onset
GH deficiency. European Journal of Endocrinology 2009; 160: 899-907.
- Pituitary Foundation information leaflet `The Pituitary Gland' 2012.
http://www.pituitary.org.uk/information/publications/essential-free-publications/hydrocortisone-advice-pituitary-patient-leaflet/
This leaflet has been downloaded or distributed as hard copies
6,297 times in the last three years.
- Levy,M. Interview with Peter Sonksen. Endocrinologist 2013: Spring; page
6-9.
http://www.endocrinology.org/endocrinologist/107/107.pdf#page=6