The development of a novel class of anticancer drugs, PARP inhibitors, has attracted multi-million dollar investments in clinical trials by nine pharmaceutical companies
Submitting Institution
Newcastle UniversityUnit of Assessment
Clinical MedicineSummary Impact Type
TechnologicalResearch Subject Area(s)
Biological Sciences: Biochemistry and Cell Biology
Medical and Health Sciences: Oncology and Carcinogenesis
Summary of the impact
Newcastle research selected the DNA repair enzyme poly(ADP-ribose)
polymerase (PARP) as a promising target for cancer therapy. The
first-in-class PARP inhibitor, rucaparib, was developed at Newcastle, in
collaboration with Cancer Research UK and Agouron Pharmaceuticals, and
subsequently became the first PARP inhibitor to be used to treat a cancer
patient in a clinical trial. Currently, at least 8 PARP inhibitors are
being developed and major pharmaceutical companies have to date invested
around $385 million in clinical trials, and over 7,000 patients worldwide
have been treated with PARP inhibitors in trials since 2008, demonstrating
the importance of basic and translational research in universities to drug
discovery by pharmaceutical companies.
Underpinning research
Key Newcastle researchers and their roles at the time of the research
(Where people left/joined the university in the period 1993-2013, years
are given in brackets)
AH Calvert (1990-2009), professor of medical oncology; NJ Curtin,
lecturer/senior lecturer 1998- 2006, then professor of experimental cancer
therapeutics; BW Durkacz (1982-2010) was the project originator; she was a
reader 1984-2008, then professor of experimental cancer therapeutics; BT
Golding, professor of organic chemistry 1983-2006, then senior research
investigator; RJ Griffin, reader in cancer therapy 1991-2001, then
professor of medicinal chemistry; DR Newell, professor of cancer
therapeutics; R Plummer (2001 onwards), clinical lecturer 2001- 2004,
clinical senior lecturer of oncology 2004-2008, then clinical professor of
experimental cancer medicine.
Background
DNA repair pathways can enable cancerous cells to survive the DNA damage
induced by radiation therapy and chemotherapy. Thus, inhibitors of these
pathways could enhance the effect of these treatments. Basic research at
Newcastle instigated by Prof Barbara Durkacz selected the DNA repair
enzyme poly(ADP-ribose) polymerase (PARP) as a promising target for cancer
therapy. Multiple pathways contribute to the repair of DNA and PARP is a
key enzyme in the repair pathway. Early PARP inhibitors, the benzamides,
were developed in the 1980s, but lacked the potency and specificity
required for pre-clinical evaluation.
Research
Since 1995, the work of a multidisciplinary team at
Newcastle has resulted in the development of novel and potent PARP
inhibitors (1000 times more potent than benzamides) that selectively
inhibit the enzyme [R1, R2, R3]. These were developed using
structure-based drug design, in collaboration with Agouron Pharmaceuticals
and Cancer Research UK. The chemo- and radio- potentiating abilities of
these inhibitors were evaluated in animal models and cell cultures and
they were demonstrated to have a cellular activity that increases the DNA
damage induced by cytotoxic anticancer drugs and ionising radiation [e.g.
R2].
Cancer Research UK selected the potent PARP inhibitor rucaparib
(AG014699, CO-338) for clinical trials, and the first cancer patients in
the world to receive a PARP inhibitor were treated at Newcastle in 2003 as
part of a Phase I study [R4]. With 33 patients, the study demonstrated
that rucaparib in combination with the chemotherapeutic drug temozolomide,
was well tolerated by patients, and confirmed PARP inhibition in all
patients [R4]. Subsequently a Phase II study with 40 patients demonstrated
that temozolomide efficacy was increased when used in combination with
rucaparib [R5].
In parallel, the research undertaken at Newcastle stimulated widespread
interest, both in industry and academia, in PARP as a target in cancer
therapies, with more than 10 compounds subsequently selected for
development. In collaboration with Sheffield (Prof Thomas Helleday), the
Newcastle group also demonstrated the synthetic lethality of PARP
inhibitors towards cells with mutations in the BRCA genes, the underlying
cause of many inherited breast and ovarian cancers [R3, R6]. Synthetic
lethality is defined as the lethal effect of inactivating two enzymes or
pathways when inactivation of either alone is tolerated [6].
References to the research
(Newcastle researchers in bold. Citation count from Scopus, July 2013)
R1. Griffin RJ, Srinivasan S, Bowman K, Calvert
AH, Curtin NJ, Newell DR, Pemberton LC, Golding
BT. Resistance-modifying agents. 5. Synthesis and biological
properties of quinazolinone inhibitors of the DNA repair enzyme
poly(ADP-ribose) polymerase (PARP). (1998) Journal of Medicinal Chemistry,
41(26):5247-56. DOI: 10.1021/jm980273t. Cited by 79
R2. Calabrese CR, Almassy R, Barton S, Batey MA,
Calvert AH, Canan-Koch S, Durkacz BW, Hostomsky Z, Kumpf
RA, Kyle S, Li J, Maegley K, Newell DR, Notarianni E,
Stratford IJ, Skalitzky D, Thomas HD, Wang LZ, Webber SE,
Williams KJ, Curtin NJ. Anticancer chemosensitization and
radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor
AG14361. (2004) Journal of the National Cancer Institute, 96:56-67. DOI:
10.1093/jnci/djh005. Cited by 216
R3. Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez
E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific
killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose)
polymerase. (2005) Nature, 434:913-917. DOI:10.1038/nature03443. Cited
by 982
R4. Plummer R, Jones C, Middleton M, Wilson R, Evans J,
Olsen A, Curtin N, Boddy A, McHugh P, Newell D,
Harris A, Johnson P, Steinfeldt H, Dewji R, Wang D, Robson L, Calvert
H. Phase I study of the poly(ADP-ribose) polymerase inhibitor,
AG014699, in combination with temozolomide in patients with advanced solid
tumors. (2008) Clinical Cancer Research, 14:7917- 7923. DOI:
10.1158/1078-0432.CCR-08-1223. Cited by 128
R5. Plummer R, Lorigan P, Steven N, Scott L, Middleton MR, Wilson
RH, Mulligan E, Curtin N, Wang D, Dewji R, Abbattisya A,
Gallo J, Calvert H. A phase II study of the potent PARP inhibitor,
Rucaparib (PF-01367338, AG014699), with temozolomide in patients with
metastatic melanoma demonstrating evidence of chemopotentiation (2013)
Cancer Chemotherapy Pharmacology, 71:1191-1199. DOI:
10.1007/s00280-013-2113-1. (Published in May 2013; not yet cited)
R6. Drew Y, Mulligan EA, Vong WT, Thomas HD,
Kahn S, Kyle S, Mukhopadhyay A, Los G, Hostomsky Z,
Plummer ER, Edmondson RJ, Curtin NJ. Therapeutic
potential of poly(ADP- ribose) polymerase inhibitor AG014699 in human
cancers with mutated or methylated BRCA1 or BRCA2. (2011) Journal of the
National Cancer Institute, 103:334-346. DOI: 10.1093/jnci/djq509. Cited
by 47
Selected funding awards
• 1993-1996 The synthesis and evaluation of inhibitors of poly-ADP
ribose polymerase and nucleoside transport to potentiate the activity of
cytotoxic drugs. The North of England Cancer Research Campaign —
£72,000.
• 1998-2002 An investigation into the interactive effects of poly
(ADP-ribose) polymerase and DNA-dependent protein kinase. CRUK —
£70,016
• 1998-1999 Poly (ADP) Ribose Polymerase Inhibitors. Agouron
Pharmaceuticals — £531,956.
• 2001-2002 Development of pharmacodynamic assays for the clinical
evaluation of novel PARP inhibitors, and pre-clinical investigations of
backup compounds. Agouron Pfizer GRD — £220,000
• 2002-2003 NECRC Cancer Research Unit Core Grant. Cancer
Research UK — £504,404 • 2002-2005 Phase 1 Trial of the Novel PARP
Inhibitor, AG14699, in Combination with Temozolomide. CRUK —
£178,595
• 2007-2010 Therapeutic potential of PARP inhibitors in cancers
defective in BRCA1, BRCA2 or other defects contributing to a BRCAness
phenotype. Pfizer Inc. USA — £104,940.61
Details of the impact
The research initiated at Newcastle in the 1990s not only led to the
first in class trial of a PARP inhibitor but also played a key role in
establishing the translational research routes of development of this
class of agents. When the project was first established, PARP was not
considered a viable target, particularly by the pharmaceutical industry,
but the Newcastle team championed it and drove the project to clinical
proof-of-principle. PARP has now been adopted as a key cancer drug target
by the global pharmaceutical industry, and has reached cancer patients
across Europe, the Americas, Australasia and Asia, with eight PARP
inhibitors currently in clinical trial development worldwide and at least
eight cancer types being treated through clinical trials [EV a].
In 2010, Cancer Research UK formally recognised the research underpinning
the discovery and development of PARP inhibitors, awarding their inaugural
Translational Cancer Research Prize to the Newcastle PARP team. This prize
was awarded `...in recognition of the discovery and development of
novel PARP inhibitors, specifically the achievement of the team in
driving an initial scientific concept through medicinal chemistry and
preclinical work, to first-in-man clinical studies.' [EV b]. The
successful exploitation of PARP as a drug target builds on many decades of
basic research on DNA damage and repair by many scientists and clinicians.
In so doing, it demonstrates the importance of academia as a resource for
new targets in drug discovery. Notably, numerous other DNA damage and
repair targets are now being evaluated; based largely on the PARP
inhibitor paradigm.
Commercial Impact
The research has had a significant impact on the UK and global
pharmaceutical industry, with the following companies investing heavily in
clinical trials and clinical PARP inhibitor programmes: AstraZeneca,
Clovis, SanofiAventis, Abbott, Merck, Biomarin, Eisai, Cephalon and
Genentech [EV a]. It is clear that since the initial Newcastle trial
(2003-2005), in which patients were treated with a PARP inhibitor for the
first time, and the demonstration of synthetic lethality in BRCA-deficient
cancers, there has been a marked increase in the commencement of trials
testing PARP inhibitors [data extracted from EV a]:
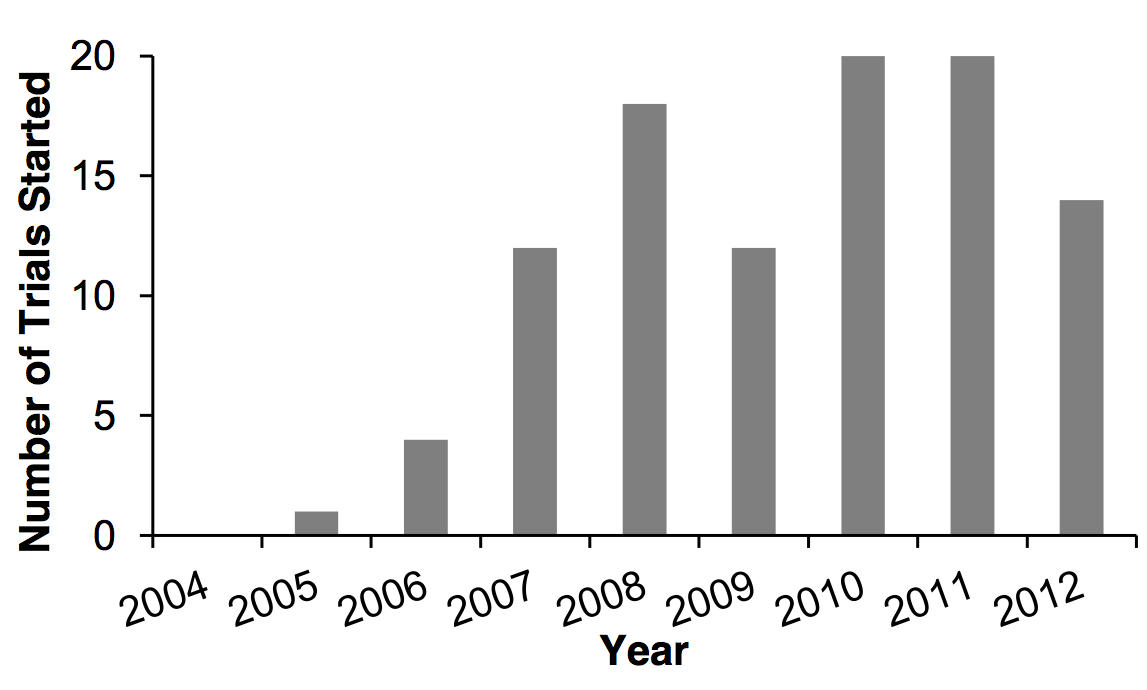
In the period May 2008- May 2013, 33 cancer trials involving PARP
inhibitors were completed and an additional 52 trials are currently open,
totalling 50 Phase I, 33 Phase II and 2 Phase III trials in this period
[EV a]. In 2011 the average per patient cost associated with a Phase I, II
and III trial in Oncology were reported to be $21,883, $73,303 and $65,900
respectively [EV c]. An estimate of the investment by companies into PARP
inhibitor trials is summarised in the following table:
| Phase |
No.
of Trials |
No.
of patients |
Average
Total cost |
| I |
50 |
3,173 |
$69.4 million |
| II |
33 |
3,160 |
$231.6 million |
| III |
2 |
1,299 |
$85.6 million |
Impact on Patients
Since the initial Phase I trial (2003), clinical trials involving PARP
inhibitors have enrolled around 7,000 patients (approx. 750 of which were
recruited to more than one trial phase), with around 5,600 patients having
enrolled in trials opening January 2008 onwards [EV a, d]. From the
outset, the potential of PARP inhibitors was clear and two out of the 33
patients treated for malignant melanoma in the Phase I trial and five out
of the 40 patients treated in the Phase II trial (2005) (both outlined in
Section 2) are today (October 2013) alive and in remission [EV e]. When
recruited into the trials, all of these patients were diagnosed with
incurable disease with a life expectancy of just a few months.
A recent Phase II trial of the PARP inhibitor olaparib in BRCA-deficient
advanced breast cancer has shown not only that this drug is well
tolerated, but also a significant reduction in tumour size in 38% of
patients (9 of 24 patients) [EV f]. Similarly, a Phase II trial showed
that this drug was well tolerated in BRCA-deficient ovarian cancer
patients, with 33% (11 of 33 patients) showing reduced tumour size [EV g].
BRCA proteins play a major role in the response to and repair of
DNA double strand breaks through the homologous recombination repair
pathway, while PARP inhibitors play a crucial role in DNA single-strand
break repair. Harmful mutations in BRCA genes produce a hereditary
breast-ovarian cancer syndrome in affected families. According to the
National Cancer Institute between 1 in 400 and 1 in 800 women will have a
BRCA mutation, which equates to a conservative estimate of around
40,000 women (1 in 800) in the UK; of these approx. 60% (24,000 women)
will develop breast cancer, and 15-40% (6,000-16,000 women) will develop
ovarian cancer. The PARP inhibitor olaparib could therefore have a
significant impact on the lives of women diagnosed with breast- or ovarian
cancer. Furthermore, PARP inhibitors offer the potential for
chemo-prevention, thereby allowing breast cancer patients to avoid
disfiguring surgery such as bilateral mastectomy and oophorectomy [EV d].
In addition, a recent small study demonstrated promising results in
patients with BRCA mutations after treatment with a new PARP inhibitor,
BMN 673; 18 out of 42 (42%) patients with ovarian or breast cancer showed
signs of tumour shrinkage after treatment [EV f].
Sources to corroborate the impact
EV a. www.clinicaltrials.gov
(Search term `PARP inhibitor',
excluding withdrawn and terminated trials. For patient
numbers, trials not yet recruiting were also excluded.)
EV b. Inaugural Cancer Research UK Translational Research Team Prize in
2010
http://www.cancerresearchuk.org/science/funding/find-grant/all-funding-schemes/translational-cancer-research-prize/past-winners/
EV c. http://www.pharmalive.com/clinical-trial-costs-are-rising-rapidly
EV d. Plummer R. Perspective on the pipeline of drugs being developed
with DNA damage as a target. Clinical Cancer Research (2010) 16,
4527-4531. DOI: 10.1158/1078-0432.CCR-10- 0984
EV e. Patient survival data; corroborating e-mail.
EV f. Tutt, A et al. Phase II trial of the oral PARP inhibitor olaparib
in BRCA-deficient advanced breast cancer. Journal of Clinical Oncology,
2009 ASCO Annual Meeting Proceedings (Post-Meeting Edition). Vol 27, No
18S (June 20 Supplement).
http://meeting.ascopubs.org/cgi/content/abstract/27/18S/CRA501
EV g. Audeh, MW et al. Phase II trial of the oral PARP inhibitor olaparib
(AZD2281) in BRCA- deficient advanced ovarian cancer. Journal of Clinical
Oncology, 2009 ASCO Annual Meeting Proceedings (Post-Meeting Edition). Vol
27, No 15S (May 20 Supplement).
http://meeting.ascopubs.org/cgi/content/abstract/27/15S/5500