Reducing the toxicity of pemetrexed treatment in malignant pleural mesothelioma.
Submitting Institution
Newcastle UniversityUnit of Assessment
Clinical MedicineSummary Impact Type
HealthResearch Subject Area(s)
Medical and Health Sciences: Clinical Sciences, Public Health and Health Services
Summary of the impact
Malignant pleural mesothelioma (MPM) is a treatable but incurable cancer
that originates in the
cells lining the lungs. Over 14,000 people worldwide are diagnosed
annually with MPM. Antifolates
are often used in cancer therapy, but side effects are a major issue. A
retrospective analysis of
cancer trials and phase 1 trial of MPM patients, carried out by Newcastle
in collaboration with Eli
Lilly Pharmaceuticals, determined that plasma homocysteine levels were a
good predictor of drug
toxicity in cancer patients treated with the antifolate pemetrexed, and
that this drug was well
tolerated by patients with low homocysteine levels. It was also determined
that pemetrexed
treatment should be supplemented with vitamin B12 as well as folic acid,
to reduce drug toxicity.
Ultimately, this permitted the continued development of pemetrexed, which
otherwise would have
been too toxic for clinical use. It is now the only licensed drug for MPM
treatment in combination
with platinum-based chemotherapy.
Underpinning research
Key Newcastle researchers
(Where people left/joined the university in 1993-2013, years are given in
parentheses)
AV Boddy (1998 onwards), lecturer/senior lecturer 1998-2006, then
professor of cancer
pharmacology; AH Calvert (1990-2009), professor of medical oncology; NJ
Curtin, lecturer/senior
lecturer 1998-2006, then professor of experimental cancer therapeutics; DR
Newell, professor of
cancer therapeutics; R Plummer (2001 onwards), clinical lecturer/senior
lecturer of oncology 2001-2008,
and then clinical professor of experimental cancer medicine.
Background
Malignant pleural mesothelioma (MPM) is a cancer that originates in the
pleura (the lining of the
lungs). Over 14,000 people worldwide are diagnosed annually with this
incurable disease, which is
most often caused by past exposure to asbestos. It is therefore
particularly prominent in
industrialised regions closely connected with shipbuilding, mining, and
construction.
Antifolates are drugs that are toxic to rapidly dividing cells such as
malignant cells; therefore, many
are used in cancer therapy. However, a major problem with antifolates is
significant side effects,
which include severe bone marrow suppression (leading to reduced immunity
and thus increased
risk of infection) and gastrointestinal toxicity: a combination that
carries a high mortality risk.
Research
Antifolate use in cancer treatment has been a major research area for
some time. Newcastle
research into the cellular activity of a multitargeted antifolate
(LY231514), now known as
pemetrexed or Alimta [e.g. R1], led to supportive laboratory studies being
performed and an
important early-phase clinical trial of pemetrexed.
Pemetrexed prevents cell replication by interfering with folate-dependent
processes; thus, it also
affects normal cells; side effects include low white and red blood cell
counts, nausea, fatigue,
shortness of breath, and anaemia. It was recognised that the ability to
predict patients more likely
to experience drug-associated toxicity could lead to significant
improvements in the management
of this problem. Thus, in a collaborative study, Newcastle and Eli Lilly
Pharmaceuticals
retrospectively analysed plasma samples from 246 patients treated with
pemetrexed, combined
with folic acid in Phase I and II trials (1995) for cancers other than
malignant pleural mesothelioma.
The aim was to identify potentially predictive factors of severe drug
toxicity [R2]. This analysis
identified a positive correlation between plasma homocysteine levels and
pemetrexed toxicity,
suggesting that measuring pre-treatment homocysteine levels could identify
patients likely to
experience severe toxicity. These findings were incorporated into the
protocol of an Eli Lilly-sponsored
Phase I clinical trial of MPM therapy, which began at the same time as the
retrospective
analysis [R3]. This trial determined the safe dose of pemetrexed with
carboplatin. The study
enrolled patients with malignant pleural mesothelioma, and was the first
prospective study to use
homocysteine levels as a marker to predict the potential adverse toxicity
of an antifolate [R3]. This
ensured that patients that were particularly vulnerable to pemetrexed
toxicity could be excluded
from the trial, leaving 27 eligible patients out of 40. The trial proved
that pemetrexed was well
tolerated in the trial patients at 500 mg/m2 body surface area.
There was also a substantial clinical
benefit: significant tumour responses (size decrease) and rapid marked
improvement of debilitating
symptoms, e.g. shortness of breath and chest pain, in 84% of the patients
[R3]. The Newcastle
Phase l trial demonstrated that pemetrexed could be administered safely to
patients in a platinum
chemotherapy combination and, ultimately this was the first therapy to be
approved by the Food
and Drug Administration (FDA) and the European Medicines Agency (EMEA) in
2004 for the
treatment of malignant pleural mesothelioma.
At the time of the retrospective analysis and Phase I trial, it was known
that folic acid
supplementation could potentially reduce antifolate toxicity, but the
underlying mechanism was
unclear. However, the retrospective analysis, which included vitamin
deficiency markers from
patients, also demonstrated that high pemetrexed toxicity correlated with
low folate and vitamin
B12 levels [R2]. As folates and vitamin B12 are required for homocysteine
metabolism, this offered
an explanation of why increased homocysteine levels were correlated with
increased drug toxicity;
gastrointestinal pathology is thought to be present in the majority of
patients with B12 deficiency. It
was therefore inferred that these patients, with their background of
gastrointestinal pathology might
experience greater antifolate toxicity, as antifolates also have
deleterious gastrointestinal side
effects. These findings suggested that pemetrexed treatment should not
only be supplemented
with folic acid, but also vitamin B12, to reduce the toxic side effects of
antifolates [R2]. Preliminary
data of vitamin B12 intervention in a Phase II pemetrexed trial confirmed
that administering folic
acid and vitamin B12 reduced homocysteine levels and in turn significantly
reduced the toxicity
associated with pemetrexed therapy while maintaining, or even improving,
efficacy [R2].
As a direct consequence of the retrospective analysis, all patients given
pemetrexed in
mesothelioma trials were also subsequently given folic acid and vitamin
B12. Eli Lilly
Pharmaceuticals supported a large multi-centre Phase III clinical trial
and Newcastle was one of
the participating centres [R4]. The protocol for this trial, which
involved 456 patients, was revised in
December 1999 to include supplementation treatment, thus 117 patients were
not given folic acid
and vitamin B12 and 339 patients were. This trial, which also incorporated
the pemetrexed dose of
500 mg/m2 body surface area, demonstrated the benefit of
combining pemetrexed with cisplatin
(another platinum drug similar to carboplatin) in patients with malignant
pleural mesothelioma. It
also confirmed that the addition of folic acid and vitamin B12
significantly reduced toxicity without
affecting drug efficacy [R4].
References to the research
(Newcastle researchers in bold. Citation count from Scopus, July 2013)
R1. Smith PG, Marshman E, Newell DR, Curtin
NJ. Dipyridamole potentiates the in vitro activity
of MTA (LY231514) by inhibition of thymidine transport. Br J Cancer. 2000
Feb;82(4):924-30.
DOI: 10.1054/bjoc.1999.1020. Cited by 23.
R2. Niyikiza C, Baker SD, Seitz DE, Walling JM, Nelson K, Rusthoven JJ,
Stabler SP, Paoletti P,
Calvert AH, Allen RH. Homocysteine and methylmalonic acid: markers
to predict and avoid
toxicity from pemetrexed therapy. Mol Cancer Ther. 2002 May;1(7):545-52.
PMID: 12479273
Cited by 186.
(Prof Calvert was senior co-author for this paper and provided the
oncology and antifolate
expertise. He was a major driver of the study and a long-standing advisor
to Eli Lilly for the
development of pemetrexed and other antifolates.)
R3. Hughes A, Calvert P, Azzabi A, Plummer R,
Johnson R, Rusthoven J, Griffin M, Fishwick
K, Boddy AV, Verrill M, Calvert H. Phase I
clinical and pharmacokinetic study of pemetrexed
and carboplatin in patients with malignant pleural mesothelioma. J Clin
Oncol. 2002
Aug;20(16):3533-44. DOI: 10.1200/JCO.2002.10.073. Cited by 118.
R4. Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie
P, Gatzemeier U,
Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P. Phase III Study of
Pemetrexed in
Combination With Cisplatin Versus Cisplatin Alone in Patients with
Malignant Pleural
Mesothelioma. J Clin Oncol. 2003; 21(14):2636-2644. DOI:
10.1200/JCO.2003.11.136. Cited
by 1149.
Selected Funding Awards
- 1999-2003 NECRC Cancer Research Unit Core Grants 2-5. Cancer
Research UK- £3,427,742
Details of the impact
It is estimated that over 14,000 people are diagnosed worldwide with
malignant pleural
mesothelioma each year, around 2,500 of which are in the UK. This disease
most commonly
develops between the ages of 50 and 70 years, affecting five times more
men than women.
Symptoms include shortness of breath, chest pains, fatigue, and weight
loss. There is no cure and
the prognosis is poor; over 2,000 people die annually from the disease in
the UK.
Impact on Patients
If not for the Newcastle research and collaboration with Eli Lilly
Pharmaceuticals, pemetrexed
toxicity, which includes severe bone marrow suppression, vomiting,
fatigue, shortness of breath,
and anaemia, would have led to its clinical development being discontinued
[EV a]. Instead, it was
demonstrated for the first time that pemetrexed could be used safely in
patients and that the
toxicity and side effects could be reduced by supplementation with not
only folic acid, but also
vitamin B12. A Vice President of Eli Lilly at the time the research was
carried out confirms that
during their collaborative work with the Newcastle group:
`...the identification of biomarkers related to folic acid and other
vitamins [including
homocysteine] has been fundamental to understand the toxicity of
Pemetrexed. High levels of
homocysteine were associated to higher risk of developing severe
toxicity and in some
instances toxic deaths. The supplementation of folic acid and vitamin
B12, currently part of the
label of Pemetrexed commercialized with the name of Alimta is the result
of this collaboration'
[EV a].
Continuing, he says that this ultimately permitted `...the completion
of the development of
Pemetrexed' [EV a]. The supplementation treatment means that
patients are better able to tolerate
the drug and stand to benefit from this treatment.
Following the Newcastle Phase I trial, the collaborative retrospective
study with Eli Lilly, and
incorporating vitamin B12 supplementation in a Phase II trial [R2, R3,
Section 2], a Phase III trial
[R4, Section 2] included the supplementation treatment and 500 mg/m2
body surface pemetrexed.
This is recognised in the 2008 National Institute for Health and Care
Excellence (NICE) guidelines
for the treatment of malignant pleural mesothelioma, which states that `...with
effect from the date
of the protocol change, all patients received supplementation' [EV
b, p. 8]. The trial confirmed that
when pemetrexed was supplemented with folic acid and vitamin B12,
incidences of severe toxicity,
which include drug-related death, neutropenia (white blood cell
reduction), febrile neutropenia, and
diarrhoea, were significantly reduced, compared to when it was not [R4,
Section 2; EV b].
Supplemented pemetrexed treatment combined with cisplatin also resulted in
a significant increase
in patients' quality of life by reducing disease symptoms, including pain,
fatigue, anorexia, and
cough [R4, Section 2; EV b].
Using pemetrexed with cisplatin also resulted in a significant survival
benefit for patients with
malignant pleural mesothelioma. In fully supplemented patients with
advanced disease, median
survival was 13.2 months when pemetrexed and cisplatin were administered,
versus 8.4 months
for patients given cisplatin alone [EV b]. Pemetrexed also increased
tumour response rates to
cisplatin (41.3% with pemetrexed versus 16.7%
without) and the median time to progressive disease
(defined as at least 20% growth in tumour size since
the start of treatment) was significantly longer for
patients who received pemetrexed and cisplatin as
compared with patients who received just cisplatin (5.7
months versus 3.9 months, respectively) [EV b].
Notably, there has been a marked increase in the
number of cancer (predominantly MPM) patients in the
UK receiving pemetrexed treatment following the
release of the 2008 NICE guidelines (bar chart) [EV c].
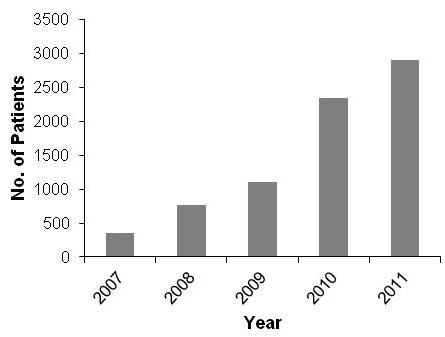
Impact on Clinical Practice
In accordance with the 2008 NICE guidelines [EV b, p. 8], pemetrexed with
cisplatin is currently the
only chemotherapy regimen licensed for treatment of malignant pleural
mesothelioma. The
guidelines cite the Phase III trial [R4, Section 2] which adopted vitamin
B12 with folic acid
supplementation into its protocol as a result of the collaborative work
between Newcastle and Eli
Lilly [R2, Section 3] as the only identified randomised controlled
pemetrexed trial in malignant
pleural mesothelioma. The NICE guidelines clearly state that `...in
order to reduce toxicity, patients
treated with pemetrexed must receive folic acid and vitamin B12
supplementation' [EV b, p. 6]. This
is also clearly stated in the 2004 FDA and EMEA approvals for pemetrexed
(Alimta) and in its
prescription information, and the maximum tolerated dose of 500 mg/m2
body surface area [EV d].
Notably, the US National Guideline Clearinghouse website provides a link
to the NICE guidelines
[EV e]. A Phase II trial on the treatment of patients with malignant
pleural mesothelioma with
pemetrexed and carboplatin, recently reported that 70% of the 76 patients
enrolled exhibited
clinical improvement after just two courses and that the pemetrexed dose
of 500 mg/m2 body
surface area was well tolerated [EV f].
The former Vice President of Eli Lilly states that to date:
`...Alimta has been used globally by hundreds of thousands of patients
and it is standard of
care in [mesothelioma and non small cell lung cancer]. The
collaboration [between Newcastle
University and Eli Lilly] has been crucial for the advancement of the
knowledge in this field and
the achievement of the introduction of a medicine that changed the
modality of treatment for
mesothelioma and lung cancer' [EV a].
Pemetrexed treatment with folic acid and vitamin B12 supplementation was
also adopted into the
NICE guidelines for non-squamous non-small cell lung cancer (comprises
most lung cancers) in
December 2010 [EV g] and for maintenance treatment of this disease in 2012
[EV h].
Clinical Trials
There are currently 361 clinical trials (either open or completed in
2008-2013) registered on
clinicaltrials.gov that use pemetrexed in accordance with the FDA and Eli
Lilly guidelines. These
include trials on malignant pleural mesothelioma, non-small cell lung
cancer, squamous cell head
and neck cancer, advanced urothelial carcinoma, ovarian carcinoma, and
thyroid cancer, and
involve over 58,000 patients [EV i].
Sources to corroborate the impact
EV a. Testimonial letter: Former VP of Eli Lilly Pharmaceuticals (Letter
held at Newcastle).
EV b. NICE technology appraisal guidance 135 (2008): Pemetrexed for the
treatment of
malignant pleural mesothelioma. http://guidance.nice.org.uk/TA135/Guidance/pdf/English
EV c. Patient numbers extracted from NHS prescription data for
pemetrexed:
http://www.hscic.gov.uk/searchcatalogue?q=pemetrexed&area=&size=10&sort=Relevance
in conjunction with statement in NICE guidelines on cost/patient for a
course of pemetrexed
treatment (EV a, p. 7).
EV d. Prescription information: http://www.lilly.com/products/human/Pages/human.aspx
EV e. http://guideline.gov/search/search.aspx?term=pemetrexed
EV f. Castagneto, B et al. Phase II study of pemetrexed in combination
with carboplatin in
patients with malignant pleural mesothelioma (MPM). Ann Oncol. 2008,
19:370-3. DOI:
10.1093/annonc/mdm501.
EV g. NICE technology appraisal guidance 181 (2010): Pemetrexed for the
first-line treatment of
non-small-cell lung cancer. PDF at:
http://guidance.nice.org.uk/TA181/Guidance/pdf/English
EV h. NICE technology appraisal guidance 190 (2012): Pemetrexed for the
maintenance
treatment of non-small-cell lung cancer. PDF at:
http://guidance.nice.org.uk/TA190/Guidance/pdf/English
EV i. Data extracted from: http://www.clinicaltrials.gov
(Search words `pemetrexed' `alimta', `500
mg/m2`; show results for trials open or completed 2008-2013)