Optimising the treatment of childhood cancer through therapeutic drug monitoring
Submitting Institution
Newcastle UniversityUnit of Assessment
Clinical MedicineSummary Impact Type
HealthResearch Subject Area(s)
Medical and Health Sciences: Clinical Sciences, Oncology and Carcinogenesis, Pharmacology and Pharmaceutical Sciences
Summary of the impact
Clinical pharmacology studies conducted at Newcastle have led to
optimisation of the administration of the chemotherapy drug carboplatin in
children with neuroblastoma and other cancers. The research provided the
rationale for carboplatin dosing based on patient renal function, with
individualised dosing resulting in increased drug efficacy and reduced
toxicity. This approach is now in widespread use in national and European
treatment protocols, benefitting over 2,500 children. Similar drug
monitoring approaches are being implemented for an increasing number of
important drugs. Following a recent Newcastle-led national clinical trial,
new dosing guidelines for the drug 13-cis retinoic acid have been
adopted for high-risk neuroblastoma patients across Europe.
Underpinning research
Key Newcastle researchers
(Where individuals left or joined the university in the period 1993-2013,
years are given in brackets)
AV Boddy (1998 onwards), lecturer/senior lecturer 1998-2006, then
Professor of Cancer Pharmacology; AH Calvert (1990-2009), professor of
medical oncology; DR Newell, professor of cancer therapeutics and senior
pharmacologist; ADJ Pearson (1989-2005), Professor of Paediatric Oncology;
GJ Veal (1998-onwards) research associate/senior research associate
1998-2005, research fellow 2005-2010 then lecturer/senior lecturer.
Background
Cancer is the leading cause of death from disease in children aged 1-14
years of age, accounting for approximately 20% of all deaths in this age
group in the UK. Neuroblastoma is the most common paediatric malignant
solid tumour outside the central nervous system, with approximately 100
cases diagnosed annually in the UK and around 900 cases in Europe. The
median age at diagnosis is 22 months and the most common type of
neuroblastoma is high risk neuroblastoma. This is incurable in over 50% of
cases, which in the UK accounts for approx. 15% of cancer related deaths
in childhood.
The platinum agent cisplatin has been used since the 1970s as a
chemotherapeutic drug. Whilst highly effective in the treatment of
neuroblastoma and other childhood cancers, the associated long-term
toxicity and side effects, including hearing loss (Brock et al. 2012,
PMID: 22547603) and kidney damage (Skinner et al. 2009, PMID: 19850470),
have encouraged the development of less toxic platinum analogues, leading
to the introduction of carboplatin for clinical use. Work in Newcastle and
elsewhere had led to a detailed understanding of the pharmacokinetics (the
fate of a drug from administration to the point when it is eliminated from
the body) of carboplatin in adults. However, there were essentially no
data available in children on the pharmacokinetics of carboplatin, a drug
used in induction and consolidation chemotherapy regimens for intermediate
and high-risk neuroblastoma, when Newcastle initiated these studies [R1].
Research
The Newcastle group identified two key factors relevant to
carboplatin-treatment in children. Firstly, removal of carboplatin from
the body was shown to be almost exclusively via elimination of unchanged
drug in the urine, with drug clearance found to be closely related to the
glomerular filtration rate of the patient. Secondly, exposure (a
pharmacokinetic measure that is derived from the drug concentration in the
blood and the time it remains in the body) to carboplatin was shown to be
more closely correlated than dose of drug administered to both toxicity
and clinical response (reduction in tumour size) [R1]. In order to improve
standard clinical practice and achieve target drug exposures, Newcastle
researchers devised equations to determine the most appropriate
carboplatin dose to be administered to individual patients; producing
dosing tables and developing appropriate blood sampling strategies to
facilitate the monitoring of drug levels in patients [R1, R2]. Subsequent
research led by Newcastle, as part of a national multi-centre study,
demonstrated in a randomised controlled trial that dosing patients
according to renal function resulted in more uniform drug exposure drug
[R3]. This ground-breaking research led to a shift from conventional
dosing based on body size or surface area to a more rational
individualised dosing approach based on renal function. This is
particularly pertinent in a paediatric setting, since there is significant
variation in renal function throughout childhood.
Further studies on methods for estimation of renal function in children
and the impact of total nephrectomy (kidney removal) and dialysis (removal
of waste from the blood) on the elimination of carboplatin [R4] have
allowed the drug to be administered safely in a variety of challenging
clinical settings. Perhaps most importantly, using a combination of
renal-function estimation and therapeutic drug monitoring, where
concentrations of the drug are measured in plasma to inform future dosing
decisions, the Newcastle group has pioneered an approach to the
safe use of high-dose carboplatin for resistant tumours [R5].
Since the carboplatin dosing approach was established, similar
therapeutic drug monitoring studies have been carried out with 13-cis
retinoic acid (13-cisRA) [R6]. This is a key drug used in
maintenance treatment for high-risk neuroblastoma patients. Recently
published data from a Newcastle-led national study indicated that children
who weigh less than 12kg, who receive a reduced dose of 13-cisRA,
are more likely to experience sub-therapeutic drug exposures and therefore
may be less likely to benefit from treatment [R6]. In addition, children
who are unable to swallow 13-cisRA capsules whole due to their
young age, for whom the drug has to be extracted and mixed with food, are
also at risk of experiencing low drug exposures [R6].
References to the research
(Newcastle researchers in bold. Citation count from Scopus, July 2013)
R1. Newell DR, Pearson ADJ, Balmanno K, Price L, Wyllie RA, Keir
M, Calvert AH, Lewis IJ, Pinkerton CR, Stevens MCG. Carboplatin
pharmacokinetics in children: The development of a pediatric dosage
formula. Journal of Clinical Oncology (1993). 11: 2314-2323. Cited by
104 (PMID:8246021)
R2. Ghazal-Aswad S, Calvert AH, Newell DR. A single-sample assay
for the estimation of the area under the free carboplatin plasma
concentration versus time curve. Cancer Chemotherapy and Pharmacology
(1996). 37: 429-434. DOI: 10.1007/s002800050408
Cited by 4
R3. Thomas HD, Boddy AV, English MW, Hobson R, Imeson J, Lewis I,
Morland B, Pearson ADJ, Pinkerton R, Price L, Stevens M, Newell
DR. Prospective validation of renal function-based carboplatin
dosing in children with cancer: a United Kingdom Children's Cancer Study
Group trial. Journal of Clinical Oncology (2000). 18: 3614-21. Cited
by 36 (PMID:11054434)
R4. Wright J, Boddy AV, Highley M, Fenwick J, McGill A, Calvert AH.
Estimation of glomerular filtration rate in cancer patients. British
Journal of Cancer (2001). 84: 452-459. DOI: 10.1054/bjoc.2000.1643.
Cited by 94
R5. Veal GJ, Errington J, Tilby MJ, Pearson ADJ, Foot ABM,
McDowell H, Ellershaw C, Pizer B, Nowell GM, Pearson DG, Boddy AV
on behalf of the UKCCSG Pharmacology Working Group. Adaptive dosing and
platinum-DNA adduct formation in children receiving high dose carboplatin
for the treatment of solid tumours. Br J Cancer (2007). 96: 725-731. DOI:
10.1038/sj.bjc.6603607. Cited by 14
Key funding awards
• 2000-2010 Pharmacology Studies in Paediatric Oncology, CRUK
Programme grant — £900,000
• 2004-2008 Pharmacology of retinoids in neuroblastoma, CRUK PhD
Studentship - £100,000
• 2005-2010 Academic Fellowship in patient-oriented medical research,
RCUK - £125,000
Details of the impact
As a result of the underpinning research detailed above, Newcastle has
become a leading centre for studying the pharmacology of drugs used to
treat children's cancer and so has had a significant impact on the conduct
of national and European clinical trials. Crucially, these trials equate
to standard treatment approaches for most childhood cancers, and trial
protocols act as clinical guidelines. Approximately 90% of neuroblastoma
patients are enrolled in clinical trials.
Benefits to Patients treated with Carboplatin
Newcastle is now established as the national centre for pharmacology
studies in childhood cancer, coordinating patient recruitment in 18 UK
treatment centres [EV a]. A blood testing service offered by the Newcastle
laboratory has been used routinely by 12 of the UK's major treatment
centres for children with cancer since 2008, including all of the largest
centres, e.g. Alder Hey, Birmingham Children's Hospital and Great Ormond
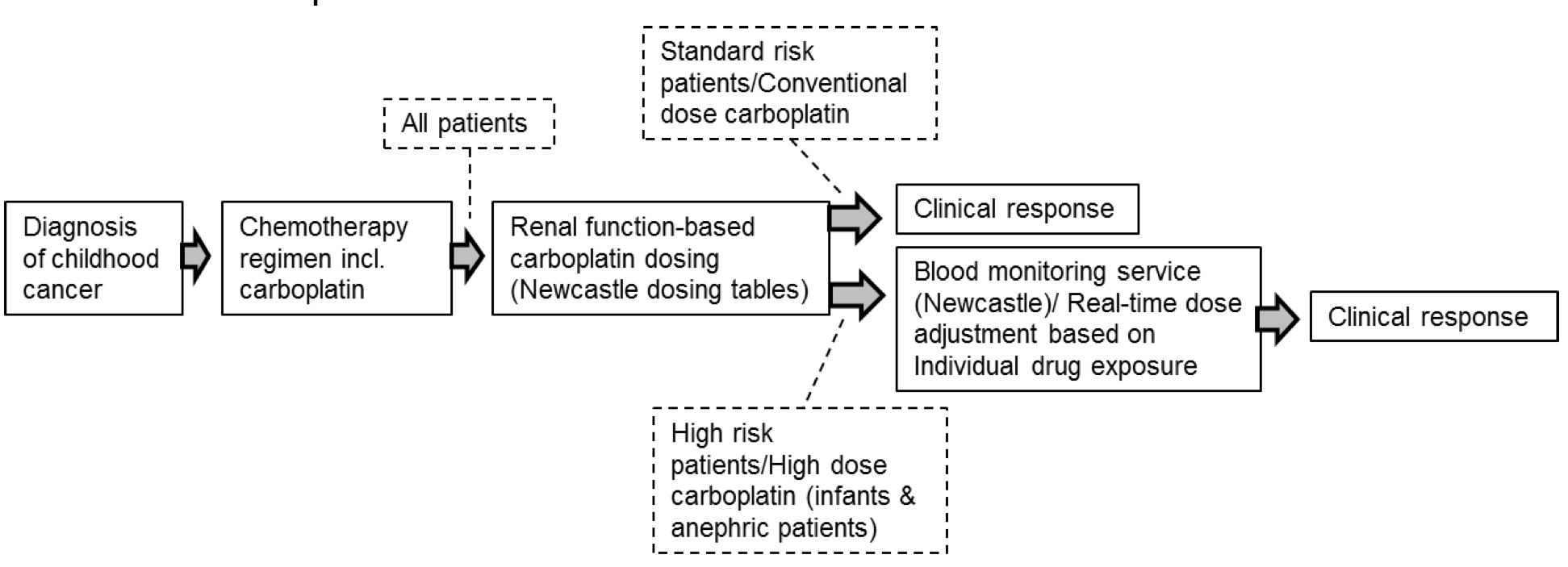
Street [EV a]. The following figure graphically represents the use of
carboplatin in treating neuroblastoma patients, in accordance with the
protocol for the European High-Risk Neuroblastoma Trial [EV b; Trial
NCT01704716] and other challenging patient populations of various tumour
types. It shows how the Newcastle blood testing service, alongside the
provision of dosing tables by the Newcastle group, has had a direct impact
on the treatment of children with carboplatin:
The blood testing service is used to determine individualised dosing for
the treatment of children with high dose carboplatin chemotherapy and
other patients where drug dosing is particularly challenging, including
very young children and those without functional kidneys, thus protecting
them from experiencing excessive drug exposures. This is a vital tool, and
one of the Consultant Paediatric Oncologists at Alder Hey hospital states
that through using this service they have `...observed the need for
significant dose adjustment in several patients' [EV c].
Furthermore, a Consultant Paediatric Oncologist at Birmingham Children's
Hospital, confirms that overdosing patients with carboplatin `...significantly
increases the chances of death from non haematological end organ
toxicity during these procedures' and that `...under dosing
patients increases the chance of relapse from their malignant tumours'
[EV d]. He goes on to say that: `...using the real-time Carboplatin
pharmacokinetically guided dosing has allowed both reductions and
increases in the predicted total dose of carboplatin of more than 20%
and this service has improved both safety and efficacy for our patients'
[EV d].
A Consultant Paediatric Oncologist at Great Ormond Street also confirms
that the Newcastle drug monitoring service `...has been invaluable for
the treatment of patients receiving high dose chemotherapy and infant
patients where the risk of drug toxicity is a real concern' [EV e].
Through involvement with the Children's Cancer and Leukaemia Group, the
Newcastle dosing tables for carboplatin are routinely included in clinical
trial protocols for various types of childhood cancer, including
neuroblastoma and brain tumours [EV b]. Between Jan 2008 and July 2013
there were four open clinical trials using the Newcastle dosing tables,
with an estimated enrolment of 2,400 children [EV b], all of whom benefit
from a more uniform drug exposure to carboplatin. In addition, since 2008
the blood testing service has been used to guide dosing in 54 children
outside of clinical trials, with dose changes implemented in approximately
75% of these patients [EV a, EV f]. Notably, the carboplatin dosage tables
have been incorporated into a European High-Risk Neuroblastoma Trial
(HR-NBL-1/SIOPEN), which to date has recruited over 2,000 children at 115
sites across Europe and Australia [EV b; Trial NCT01704716]. Furthermore,
Newcastle provides one of only three reference laboratories for this trial
[EV b; Trial NCT01704716].
As noted in Section 2, carboplatin has important advantages over cisplatin
in terms of reduced long-term toxicity. Now, thanks to the drug monitoring
approaches developed at Newcastle carboplatin-toxicity can also be
appropriately controlled, even in clinical studies where high doses of
carboplatin are necessary. This has ultimately resulted in improved care
of children being treated for a number of tumour types. Recently, use of
the Newcastle drug monitoring approach has also allowed a curative
carboplatin regimen for retinoblastoma to be used safely in the context of
maturing renal function in a neonate who was diagnosed with the disease at
35 weeks (gestational age) [EV f]. This highlighted a clinical situation
where carboplatin therapeutic drug monitoring represented the only
feasible treatment approach to ensure an appropriate drug exposure,
leading to a successful treatment outcome [EV f].
Benefits to Patients treated with 13-cis Retinoic
Acid
Since 2008, and following the establishment of the carboplatin dosing
approach, the Newcastle group have been leading therapeutic drug
monitoring studies for an additional 11 important chemotherapeutics [EV
g]. The Newcastle-led national study on dosing of 13-cis retinoic
acid (13-cisRA) in high-risk neuroblastoma patients (R6 in section
3) reported that some children are receiving potentially sub-therapeutic
doses and therefore may be less likely to benefit from treatment. 13-cisRA
is a key drug used in maintenance treatment for high-risk neuroblastoma;
combining bone marrow transplantation with 13-cisRA treatment
results in an 18% increase in 5 year survival rates compared to bone
marrow transplantation alone [EV h], highlighting the clinical importance
of this drug. As a result of the Newcastle findings, published online in
October 2012, children weighing less than 12kg now no longer receive
reduced drug doses in clinical trials across Europe [EV b; Trial
NCT01704716, EV i], and recommended increased dose levels for children
unable to swallow capsules have also been adopted [EV b; Trial
NCT01704716, EV i]. These improved dosing guidelines have an impact on
over two-thirds of high-risk neuroblastoma patients and allow for optimal
administration of 13-cisRA across Europe, as highlighted in a
recent editorial published in Clinical Cancer Research [EV j].
Sources to corroborate the impact
EV a. List of UK Centres Utilising Newcastle Real-Time Carboplatin
Monitoring / Dose Adjustment Service, including patient numbers, supplied
by the Blood Sampling Service (Contact provided, and list available on
request).
EV b. Data and protocols sourced from clinicaltrials.gov.uk.Trial refs:
NCT01704716, NCT00047138, NCT00025103 and NCT00274950
(Collated table of trials, and full protocol for NCT01704716 available on
request)
EV c. Letter from Consultant Paediatric Oncologist (Alder Hey Hospital,
Liverpool)
EV d. Letter from Consultant Paediatric Oncologist (clinical lead for
chemotherapy) (Birmingham Children's Hospital)
EV e. Letter from Consultant Paediatric Oncologist (Great Ormond Street
Hospital, London)
EV f. Picton et al. Therapeutic monitoring of carboplatin dosing in a
premature infant with retinoblastoma. Cancer Chemother Pharmacol (2009)
63:749-752. DOI: 10.1007/s00280-008-0787-6.
EV g. Literature search; trials using drug-monitoring approaches for
chemotherapeutic drugs other than carboplatin. Table, including
references, available on request.
EV h. Matthay KK et al. Long-Term Results for Children with High-Risk
Neuroblastoma Treated on a Randomized Trial of Myeloablative Therapy
Followed by 13-cis-Retinoic Acid: A Children's Oncology Group
Study. J Clin Oncol (2009). 27(7):1007-13. DOI: 10.1200/JCO.2007.13.8925.
EV i. Long Term Continuous Infusion ch14.18/CHO Plus s.c. Aldesleukin
(IL-2) (LTI):
http://www.clinicaltrials.gov/ct2/show/NCT01701479
EV j. Matthay KK. Targeted isotretinoin in neuroblastoma: Kinetics,
Genetics or Absorption. Clin Cancer Res (2013) 19(2):311-3. DOI:
10.1158/1078-0432.CCR-12-3313