NICE work: improving evidence-based clinical guideline development and implementation
Submitting Institution
Newcastle UniversityUnit of Assessment
Public Health, Health Services and Primary CareSummary Impact Type
EconomicResearch Subject Area(s)
Medical and Health Sciences: Public Health and Health Services
Summary of the impact
Clinical practice guidelines published in the UK by the National
Institute for Health and Care Excellence (NICE) are constructed
using an approach based on methodological research led by Professor Martin
Eccles of Newcastle University. This systematic approach includes the
incorporation of health economics considerations and review after three
years (and if found necessary, an update of the guidelines); both
important outcomes of Newcastle research. The implementation of guidelines
has long been an area of concern. Professor Eccles established and chaired
(2008-12) the NICE Implementation Strategy Group, which sought to improve
the assistance that NICE gives organisations in order to aid the
implementation of guideline recommendations. Valid clinical practice
guidelines, when implemented, lead to health gains and predictable care
costs, thus helping both patients and the NHS.
Underpinning research
Key Researcher
Professor Martin Eccles conceived and led the research in Newcastle and
contributed significantly to collaborative work, detailed where
appropriate.
Background / context
Clinical practice guidelines, defined as "systematically developed
statements to assist practitioner and patient decisions about
appropriate health care for specific clinical circumstances",
represent one of the foundations for efforts to improve healthcare (Field
& Lohr, National Academy Press 1990). The development of clinical
practice guidelines (hereafter referred to as guidelines) began in the USA
in the early 1990s. However, legal challenges (a result of the largely
private nature of US healthcare) stopped progress soon afterwards leaving
significant methodological issues to be addressed, particularly
validation. Guidelines are considered valid only if: "when followed,
they lead to the health gains and costs predicted for them" (Field
& Lohr, National Academy Press 1992). When appropriately disseminated
and implemented, valid guidelines can lead to changes in clinical practice
and improvements in patient outcome. Conversely, the dissemination and
implementation of invalid guidelines may lead to wasteful use of resources
on ineffective interventions or, worse still, deterioration in patients'
health. Research at Newcastle University sought to fill this gap in the
development of valid guidelines.
Research
The research undertaken at Newcastle first addressed the question of how
best to develop valid guidelines that help to improve the quality of
patient care. Building on the general method developed by the Agency for
Health Care Policy and Research in the USA, and refining it for
application to the NHS, in 1996 Professor Eccles led the development of
the first evidence-based guideline in the UK (R1). The following year,
further guidelines were published, along with a practical methodology for
the production of evidence-based guidelines (R2).
There had been little theoretical exploration, and a lack of a widely
accepted means, of incorporating economic considerations into guidelines.
The NHS Research and Development Health Technology Assessment Programme
document How to develop cost conscious guidelines (R3), authored
jointly by Eccles and Mason (York University) in 2001, was the first
published practical approach in which cost and cost-effectiveness concepts
were successfully incorporated into guideline development processes.
Between 2001 and 2005, Newcastle researchers played a leading role in an
international research effort that studied guidelines in use. This showed
that guidelines have an average lifespan of about six years and stated "As
a general rule, guidelines should be reassessed for validity every 3
years." (R4, p1461) Further research led to a publication that
described approaches for updating clinical guidelines (R5).
In addition to this focus on guideline development, Newcastle researchers
developed an understanding of how new knowledge described in academic
medical literature was taken up by practising clinicians. This research
had implications for improving the implementation of guidelines in the
clinical setting (R6).
References to the research
(Newcastle author in bold type, citation counts from Scopus, July 2013)
R1. Eccles MP and members of the guideline development and
technical advisory groups. North of England evidence based guidelines
development project: summary version of evidence based guideline for the
primary care management of asthma in adults. British Medical Journal
1996;312:762-6. doi: http://dx.doi.org/10.1136/bmj.312.7033.762
Cited by 43.
R2. Eccles MP, Clapp Z, Grimshaw JM, Adams PC, Higgins B, Purves
I, Russell IT. North of England evidence based guideline development
project: methods of guideline development. British Medical Journal
1996;312:760-2. doi:
http://dx.doi.org/10.1136/bmj.312.7033.760 Cited by 140.
R3. Eccles M, Mason J. How to develop cost-conscious guidelines. Health
Technology Assessment 2001;5(16). doi:10.3310/hta5160 Cited by
96.
R4. Shekelle P, Ortiz E, Rhodes S, Morton S, Eccles M,
Grimshaw J, Woolf S. Validity of the agency for healthcare research and
quality clinical practice guidelines: how quickly do guidelines become
outdated? Journal of the American Medical Association
2001;286:1461-7. doi: 10.1001/jama.286.12.1461 Cited by 238.
(Eccles contributed to the study concept and design, the analysis of data,
and the critical revision of the manuscript for important intellectual
content.)
R5. Eccles M, Rousseau N, Freemantle N. Updating evidence-based
clinical guidelines. Journal of Health Services Research and Policy
2002;7:98-103. doi:10.1258/1355819021927746 Cited by 15.
R6. Eccles M, Grimshaw J, Walker A, Johnson M, Pitts N. Changing
the behavior of healthcare professionals: the use of theory in promoting
the uptake of research findings. Journal of Clinical Epidemiology
2005;58:107-12. doi: 10.1016/j.jclinepi.2004.09.002 Cited by 213.
Relevant funding awards, by funder
Northern Regional Health Authority: Guidelines for management of
ischemic heart disease and asthma. 1993 for 18 months; £51,000.
NHS R&D Programme: Evaluating methods to promote the
implementation of research findings. 1997 for 42 months; £798,682.
Details of the impact
As a result of research led by Newcastle University, the UK National
Institute for Health and Care Excellence (NICE) has adopted: (i)
methods by which the world's leading valid clinical practice guidelines
are produced; and (ii) processes that offer the best opportunity for
implementation by those responsible. This maximises the opportunities for
health gains for patients and predictable costs for the NHS.
The National Institute for Health and Care Excellence
NICE was established in 1999, and over time it has expanded its remit to
provide guidance and advice to improve health and social care. NICE
guidance supports healthcare professionals and others in England to ensure
that the care they provide is of the highest quality, and that it offers
the best value for money. NICE is internationally recognised for the way
in which recommendations are developed, using the best available evidence
(Ev a).
NICE guidelines: the ongoing impact of Newcastle research
The first guideline adopted by NICE was one developed by Eccles' research
group concerning secondary prophylaxis following myocardial infarction (Ev
b). NICE also adopted the approach by which the guideline (and those on
asthma (R1) and another on angina) was produced. Six research publications
(led by the Newcastle group) on guideline development (including R3, R5
and R6 above) were later cited in the first edition of the NICE
Guideline Development Manual (2004). These publications continue to
underpin the method for preparing guidelines, as can be observed most
recently in the November 2012 edition of the NICE Guideline
Development Manual (Ev c).
A total of 174 NICE guidelines had been produced using the approved
methods as of July 2013, the majority of which were after 2007. In the
seven years 2001-7, a total of 65 guidelines were published. In the
following five years 109 guidelines were published (Ev d). Of all
guidelines produced, 169 remain in force: 142 of which are in their
original form and 27 have been updated.
A significant development has been the introduction of the requirement to
review guidelines and, if necessary, update them. This process has largely
taken place since 2008 (see Figure 1). NICE policy, which is based on
Newcastle-led work on the lifespan of guidelines, is: "A formal review
of the need to update a guideline is usually undertaken by NICE 3 years
after its publication" (Ev c, p189). This three-year recommendation
is based on advice presented in the 2001 Journal of the American
Medical Association paper (R4). Since 2008, 22 guidelines have been
re-written, and 67 other guidelines have been subjected to the review
process. Five guidelines have been withdrawn as a result of the review
process.
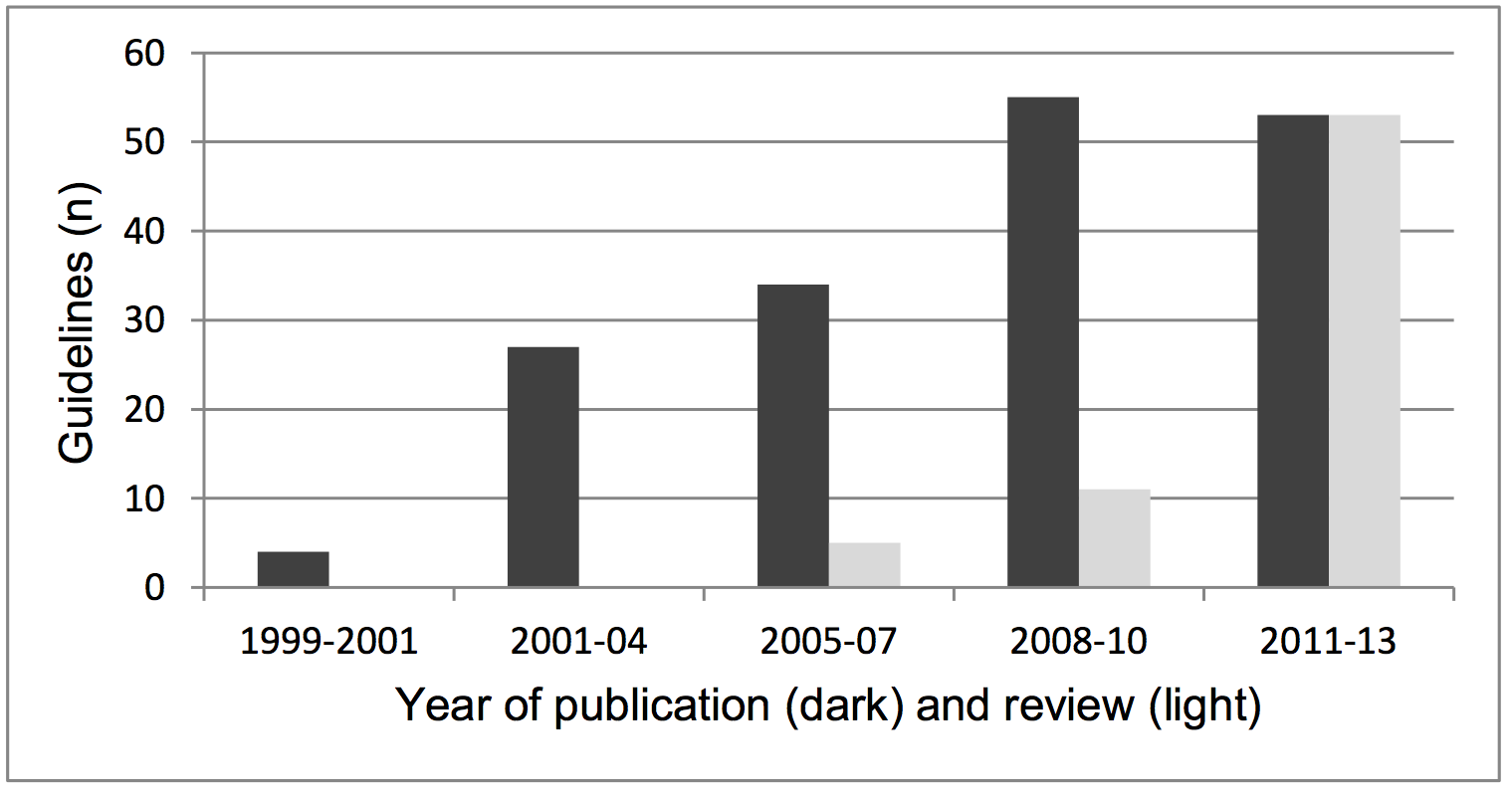 Figure 1. The number of NICE guidelines (n) published and reviewed, by three-year periods.
(Data obtained from www.nice.org.uk/guidance, Ev d)
Figure 1. The number of NICE guidelines (n) published and reviewed, by three-year periods.
(Data obtained from www.nice.org.uk/guidance, Ev d)
Advising on development and implementation
The Deputy Chief Executive of NICE has said of Professor Eccles and his
research that:
"...your work on the effectiveness of strategies to implement evidence
based guidelines [... has] informed NICE's understanding of the
evidence base for implementation and led to the decision to form NICE's
Implementation Strategy Group." (Ev e)
Eccles chaired the Implementation Strategy Group from 2008 until retiring
from it in 2012 (Ev f). Consequently, Newcastle-led research on strategies
for promoting the uptake of research findings was incorporated into the
processes and activities of NICE.
Examples:
(i) Encouraging engagement in promoting implementation. Guidelines
are, and have always been, written and reviewed by external committees
convened for that purpose, rather than NICE staff. The Implementation
Strategy Group scrutinised the processes by which guideline development
committee members were recruited. As a result of Newcastle-led research on
behaviour change amongst professionals (R6), a recommendation was made
regarding the importance of appointing the appropriate people to promote
guideline implementation. As a result, since Autumn 2009, the NICE
Implementation Team has advised management on potential gaps in guideline
development committee membership in order to encourage the recruitment of
individuals who could be expected to champion the implementation of a
guideline.
(ii) Web-based practical advice on implementation. In 2009, the
Implementation Strategy Group gave advice (based on research such as that
described in R6) on promoting engagement with primary care services and
supported the production of web pages designed to aid general
practitioners with the implementation of guidelines. The Implementation
Programme Director of NICE has said:
"This is still a well used part of the site. ... A cross Institute
group on general practice was formed and still active in 2013. [The
number of page] views within the first weeks of its launch were
equivalent to that of our most viewed guideline which is unusually high
for a support product, rather than a guidance product." (Ev g)
Sources to corroborate the impact
Ev a. NICE website http://www.nice.org.uk/aboutnice/howwework/how_we_work.jsp
Ev b. The first NICE guideline can be found at: http://guidance.nice.org.uk/A
Ev c. NICE, `The guidelines manual' Published on 30
November 2012. The web version is available at http://publications.nice.org.uk/pmg6
and a searchable pdf version (downloaded 17/06/13) is available on
request.
Ev d. The individual guidelines are available, beginning at http://www.nice.org.uk/guidance/index.jsp?action=byType&type=2&status=3
and continuing for several pages. Dates were gathered from webpages and
tabulated. This data can be made available on request.
Ev e. A letter from the Deputy Chief Executive of NICE (who has
agreed to be contacted to corroborate these claims if required) is
available on request.
Ev f. Notification of the vacancy, with a description of the role
of the Implementation Strategy Group is available at
http://www.nice.org.uk/getinvolved/joinnwc/ChairAndMemberImplementationStrategyGroup.jsp
Ev g. A copy of e-mail communication with the Implementation
Programme Director of NICE (who has agreed to be contacted if required to
corroborate the claimed impacts) is available.