Evaluating and introducing pneumococcal conjugate vaccines (PCV) into the UK infant immunisation programme
Submitting Institution
University College LondonUnit of Assessment
Clinical MedicineSummary Impact Type
HealthResearch Subject Area(s)
Medical and Health Sciences: Public Health and Health Services
Summary of the impact
A programme of work undertaken jointly between the UCL Institute of Child
Health (ICH) Vaccine Evaluation Laboratory headed by Professor David
Goldblatt and the Health Protection Agency (now Public Health England
[PHE]) led by Professor Liz Miller, has led directly to the introduction
of pneumococcal conjugate vaccines (PCV) into the UK infant immunisation
schedule. These vaccines have reduced the burden of invasive disease in
the UK saving many lives and reducing morbidity from these devastating
infections. This work has also provided the evidence for other countries
to introduce PCV with fewer than the originally recommended doses, thus
improving cost effectiveness and hastening the implementation of these
vaccines worldwide. Goldblatt has also contributed to a WHO programme to
roll out PCV in developing countries; by July 2013 this programme had
vaccinated around 10 million children.
Underpinning research
Since 1993, the Goldblatt laboratory has had a leading role in global
efforts aimed at establishing and standardising pneumococcal assays for
the purpose of assessing and licensing pneumococcal vaccines [1].
In 2002 the laboratory was designated one of only two World Health
Organisation (WHO) Reference Laboratories for Pneumococcal Serology in
recognition of its role in standardising pneumococcal assays and
establishing correlates of protection to license second generation
pneumococcal conjugate vaccines [2]. The WHO funded the laboratory
to develop assays, teach and train staff from organisations around the
world in the conduct of such assays and to transfer materials and
technology to other laboratories to facilitate global efforts to rapidly
introduce pneumococcal vaccines in areas of the world most in need. The
laboratory has just finished leading an international effort to develop a
new Pneumococcal Standard Reference serum to replace the dwindling stocks
of an existing standard. The new Standard will enable assays to be
quality-controlled for the next 50 years [3].
The first PCV, a seven-valent formulation, was licensed in 2000 but
initially only used in the USA where it was administered, according to a
four-dose schedule. In collaboration with PHE, we were the first in the
world to formally assess the utility of a three-dose, rather than the
licensed four-dose schedule and to predict the efficacy of the reduced
schedule based on the data generated in this study [4]. A
three-dose schedule was desirable in the UK to facilitate introduction
into an already crowded immunisation programme and to address the issue of
cost-effectiveness. The results of this study led directly to the decision
in the UK to introduce PCV into the infant immunisation schedule with only
three doses (September 2006).
ICH and PHE subsequently proceeded to establish the immunological basis
for the effectiveness of the response to a three-dose schedule and to
refine the understanding of pneumococcal correlates of protection [5].
Underpinning research at ICH continues to inform the evolving use of PCV.
The Goldblatt laboratory has led a study of PCV administration with the
first dose at birth, to assess safety and likely efficacy of early
vaccination in developing countries where up to a quarter of pneumococcal
deaths under the age of 2 years occur before infants are eligible for
their first vaccine [6].
References to the research
[1] Plikaytis BD, Goldblatt D, Frasch CE, Blondeau C, Bybel MJ,
Giebink GS, Jonsdottir I, Kayhty H, Konradsen HB, Madore DV, Nahm MH,
Schulman CA, Holder PF, Lezhava T, Elie CM, Carlone GM. An analytical
model applied to a multicenter pneumococcal enzyme-linked immunosorbent
assay study. J Clin Microbiol. 2000 Jun;38(6):2043-50. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC86724/
[2] Jodar L, Butler J, Carlone G, Dagan R, Goldblatt D, Kayhty H,
Klugman K, Plikaytis B, Siber G, Kohberger R, Chang I, Cherian T.
Serological criteria for evaluation and licensure of new pneumococcal
conjugate vaccine formulations for use in infants. Vaccine. 2003 Jul
4;21(23):3265-72. http://dx.doi.org/10.1016/S0264-410X(03)00230-5
[3] Goldblatt D, Plikaytis BD, Akkoyunlu M, Antonello J, Ashton
L, Blake M, Burton R, Care R, Durant N, Feavers I, Fernsten P, Fievet F,
Giardina P, Jansen K, Katz L, Kierstead L, Lee L, Lin J, Maisonneuve J,
Nahm MH, Raab J, Romero-Steiner S, Rose C, Schmidt D, Stapleton J, Carlone
GM. Establishment of a new human pneumococcal standard reference serum,
007sp. Clin Vaccine Immunol. 2011 Oct;18(10):1728-36. http://dx.doi.org/10.1128/CVI.05252-11
[4] Goldblatt D, Southern J, Ashton L, Richmond P, Burbidge P,
Tasevska J, Crowley-Luke A, Andrews N, Morris R, Borrow R, Cartwright K,
Miller E. Immunogenicity and boosting after a reduced number of doses of a
pneumococcal conjugate vaccine in infants and toddlers. Pediatr Infect Dis
J. 2006 Apr;25(4):312-9 http://dx.doi.org/10.1097/01.inf.0000207483.60267.e7
[5] Goldblatt D, Southern J, Ashton L, Andrews N, Woodgate S,
Burbidge P, Waight P, Miller E. Immunogenicity of a reduced schedule of
pneumococcal conjugate vaccine in healthy infants and correlates of
protection for serotype 6B in the United Kingdom. Pediatr Infect Dis J.
2010 May;29(5):401-5. http://dx.doi.org/10.1097/INF.0b013e3181c67f04
[6] Scott JA, Ojal J, Ashton L, Muhoro A, Burbidge P, Goldblatt D.
Pneumococcal conjugate vaccine given shortly after birth stimulates
effective antibody concentrations and primes immunological memory for
sustained infant protection. Clin Infect Dis. 2011 Oct;53(7):663-70. http://dx.doi.org/10.1093/cid/cir444
Details of the impact
Streptococcus Pneumoniae is an important cause of infection at the
extremes of life. In the United Kingdom the incidence of invasive
pneumococcal disease (IPD) is 37-48 per 100,000 for children under 1 year
and 21-36 per 100,000 for adults >65 years. In the period July 1996 to
June 2006 (prior to vaccine introduction) there were 52,579 cases of IPD
identified through the UK laboratory- based surveillance system. This does
not take into account the majority of pneumonia and otitis media cases [a].
The first pneumococcal conjugate vaccine containing seven of the most
prevalent serotypes (PCV7) was licensed in 2000 as a four-dose schedule.
Adding a new vaccine to the relatively crowded UK infant immunisation
schedule in the early 2000s presented significant difficulties, as infants
were already receiving two injections at each immunisation visit (at 2, 3
and 4 months of age). Our demonstration that three doses were
immunologically broadly equivalent to four gave the Department of Health
the evidence base to introduce PCV into the routine infant immunisation
programme vaccine with doses at 2, 4 and 12 months ("2+1" schedule). An
initial catch-up campaign was conducted in 2006/7 with routine
immunisation beginning in 2008 [b].
The introduction of PCV in the UK has had a clear impact on pneumococcal
disease. Prior to its introduction, deaths from IPD in children under five
had peaked at 797 cases/year. In 2011/12 (the last year for which there
are complete data) this had reduced by 50% to 340 deaths. Notably, cases
of IPD due to the seven serotypes included in the vaccine have fallen
dramatically, as the following table shows:
Table 1: Serotype specific IPD cases for under fives in England and Wales
prior to and following the introduction of PCV7 into the infant
immunisation programme [c]
| Serotype |
2003/4 |
2011/12 |
| 4 |
15 |
0 |
| 6B |
52 |
1 |
| 9V |
31 |
1 |
| 14 |
142 |
2 |
| 18C |
38 |
4 |
| 19F |
49 |
5 |
| 23F |
25 |
1 |
On the basis of early experience with PCV7, Goldblatt worked with
international colleagues to define the first correlates of protection and
then helped to draw up WHO guidelines for how PCVs should be subsequently
licensed (WHO TRS 927) [d]. These guidelines were used to license
10- and 13-valent formulations approved in 2009/10. In 2010 the 13-valent
vaccine was introduced in the UK. This vaccine addressed the burden of
disease caused by the additional serotypes included in the PCV13 vaccine.
In addition to the direct impact of the vaccine on disease in the
vaccinated infants, the PCV vaccine reduces the carriage of pneumococci in
the nasopharynx, which thus reduces the spread of the pneumococci and
impacts on invasive disease in other age groups. The figure below (adapted
from HPA data [c]) illustrates the impact of infant vaccination on
vaccine type IPD in all age groups in the UK.
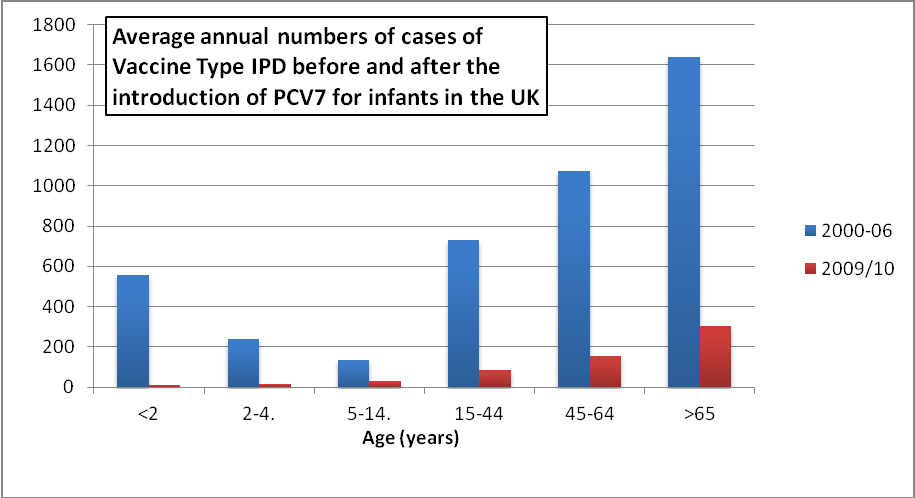
The "2+1" schedule has been highly efficacious and is also more cost
effective, as it uses fewer doses. For these reasons, many countries
worldwide have implemented it. In 2013, WHO reports that in Europe and the
Americas 23 countries are using PCV according to the schedule we developed
[e].
Our work has also contributed to global efforts to reduce mortality from
pneumococcal disease in developing countries. Half a million children
under five die each year from the condition, making it the leading
vaccine-preventable cause of death among young children. In response to
this issue, WHO and the GAVI alliance have initiated an innovative
financing process — the Advanced Market Commitment (AMC) [f] —
designed to make effective and affordable pneumococcal vaccines available
for children in developing countries. Goldblatt chaired the committee that
defined the minimum product specification (TPP) for the vaccine. This
report was published in February 2008 [g] and in March 2010,
UNICEF entered into agreements with GSK and Pfizer to supply 30 million
doses annually for 10 years [h]. An independent report into the
AMC process reported that: "Several interviewees praised the TPP for
striking an appropriate balance between setting a high bar to ensure
vaccine effectiveness and still allowing low-cost producers to compete.
The TPP also proved useful in inspiring and supporting similar guidance
for other prospective vaccines" [i]. Since 2010, over 25
countries have begun to roll out pneumococcal vaccines under this
programme; by July 2013, it was estimated that GAVI and its partners have
immunised more than 10 million children [j]. GAVI aims to increase
this to 45 countries by 2015, projecting that this will prevent more than
half a million deaths in this period.
Sources to corroborate the impact
[a] http://www.hpa.org.uk/web/HPAweb&Page&HPAwebAutoListName/Page/1203409671876.
[b] The Green Book, chapter 25:
https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/216088/Green-Book-Chapter-25-v4_0.pdf (References Goldblatt et al 2006; book page
311, pdf page 17)
[c] Data provided by Public Health England. Contact details provided.
[d].
http://www.who.int/entity/biologicals/areas/vaccines/pneumo/Pneumo_final_23APRIL_2010.pdf.
(International Reference Materials, page 6; Authors and Acknowledgements,
page 34; Reference 23, page 37).
[e] WHO vaccine-preventable diseases: monitoring system. 2013 global
summary
http://apps.who.int/immunization_monitoring/globalsummary/schedules.
[f] GAVI alliance website detailing the pneumococcal Advance Market
Commitment
http://www.gavialliance.org/funding/pneumococcal-amc/about/
[g] Target Product Profile (TPP) for the Advance Market Commitment (AMC)
for Pneumococcal Conjugate Vaccines: www.who.int/immunization/sage/target_product_profile.pdf.
(Vaccine dosage schedule page 22, Contributors page 29, References 66 and
75).
[h] GSK press release: http://www.gsk.com/media/press-releases/2010/gsk-joins-global-vaccine-
alliance-to-help-prevent-millions-of-children-from-contracting-pneumococcal-disease-in-the-worlds-poorest-countries.html
[i] The Advance Market Commitment for Pneumococcal Vaccines: Process and
Design Evaluation. February 15, 2013. Dalberg Global Development Advisors.
http://www.gavialliance.org/library/documents/gavi-documents/evaluations/amc-process-and-design-evaluation-full-report/. (quote page 12).
[j] http://www.gavialliance.org/support/nvs/pneumococcal/
Contact details
[c] Liz Miller, Public Health England. liz.miller@hpa.org.uk
[Provided [c] data on serotype specific IPD cases for under fives in
England and Wales prior to and following the introduction of PCV7 into
the infant immunisation programme]