Delivery of new methods for safer prenatal diagnosis: non-invasive testing using cell free fetal DNA in maternal blood
Submitting Institution
University College LondonUnit of Assessment
Clinical MedicineSummary Impact Type
HealthResearch Subject Area(s)
Biological Sciences: Genetics
Technology: Medical Biotechnology
Medical and Health Sciences: Public Health and Health Services
Summary of the impact
Until recently, prenatal diagnosis of genetic conditions required
analysis of fetal genetic material obtained following invasive testing,
with a risk of miscarriage. Non-invasive prenatal diagnosis (NIPD) using
cell-free fetal DNA in maternal plasma has transformed prenatal diagnosis
for many women. Testing the maternal blood sample avoids the miscarriage
risk. At UCL, we have led the implementation into clinical practice of
NIPD for serious sex-linked and autosomal dominant disorders. After a
successful application for UK Gene Testing Network (UKGTN) Gene Dossier
approval for fetal sex determination in 2011, this is now the standard of
care across the UK.
Underpinning research
Work led by Lyn Chitty at the UCL Institute of Child Health from 2005-10
was initially focussed on determining the clinical impact of NIPD for
fetal sex determination in women considering an invasive diagnostic test
because they were at risk of carrying a baby with a serious sex-linked
disorder (e.g. Duchenne muscular dystrophy) or might require dexamethasone
treatment because of a risk of congenital adrenal hyperplasia (CAH). The
established approach to prenatal diagnosis requires an invasive test (e.g.
chorionic vil ous sampling) to obtain fetal genetic material for analysis,
procedures associated with a 0.5-1% risk of miscarriage. NIPD allows
analysis of cell-free fetal DNA (cffDNA) in the blood of pregnant mothers.
Our early clinical work, funded by an EU FP6 award, clearly showed that
NIPD reduced the rate of invasive testing by 46% as well as reducing
unnecessary administration of dexamethasone to some mothers [1].
These results led to the delivery of NIPD for fetal sex determination on a
research basis from 2006.
We also established a bank of plasma samples collected from parents with
pregnancies at risk of aneuploidy or genetic disorders. This now contains
>11,000 samples and is a resource which has underpinned the
developments described here. In 2009, Chitty was awarded an NIHR programme
grant (RAPID: Reliable Accurate Prenatal non-Invasive Diagnosis) to
investigate the feasibility of wider use of cffDNA and to develop
standards for implementation into NHS clinical practice. Since then, the
RAPID team has led the development of laboratory standards and performed a
national evaluation of NIPD for fetal sex determination that demonstrated
a high sensitivity and specificity for the method [2].
We subsequently showed that it is cheaper than traditional invasive
testing [3], and that it is highly valued by patients [4].
This research formed the basis for the development of the standards
required for formal approvals necessary to implement NIPD for fetal sex
determination for serious sex-linked disorders as a clinical test. From
2009 onwards we also developed and implemented NIPD for single gene
disorders including achondroplasia, thanatophoric dysplasia, and apert
syndrome as well as developing several tests on a bespoke per patient
basis [5, 6, 7].
References to the research
[1] Hyett JA, Gardener G, Stojilkovic-Mikic T, Finning KM, Martin PG,
Rodeck CH, Chitty LS.Reduction in diagnostic and therapeutic interventions
by non-invasive determination of fetal sex in early pregnancy. Prenat
Diagn. 2005 Dec;25(12):1111-6.
http://doi.org/fbpfq9
[2] Hil M, Taffinder S, Chitty LS, Morris S. Incremental cost of
non-invasive prenatal diagnosis versus invasive prenatal diagnosis of
fetal sex in England. Prenat Diagn. 2011 Mar;31(3):267-73.
http://dx.doi.org/10.1002/pd.2680
[3] Hil M, Finning K, Martin P, Hogg J, Meaney C, Norbury G, Daniels G,
Chitty L. Non-invasive prenatal determination of fetal sex: translating
research into clinical practice. Clin Genet. 2011 Jul;80(1):68-75.
http://dx.doi.org/10.1111/j.1399-0004.2010.01533.x
[4] Lewis C, Hil M, Skirton H, Chitty LS: Non-invasive prenatal diagnosis
for fetal sex determination - benefits and disadvantages from the service
users' perspective. Eur J Hum Genet. 2012 Nov;20(11):1127-33.
http://dx.doi.org/10.1038/ejhg.2012.50
[5] Chitty LS, Griffin DR, Meaney C, Barrett A, Khalil A, Pajkrt E, Cole
TJ. New aids for the non-invasive prenatal diagnosis of achondroplasia:
dysmorphic features, charts of fetal size and molecular confirmation using
cell free fetal DNA in maternal plasma. Ultrasound Obstet Gynecol.
2011 Mar;37(3):283-9.
http://dx.doi.org/10.1002/uog.8893
[6] Lench N, Barrett A, Fielding S, McKay F, Hil M, Jenkins L, White H,
Chitty LS. The clinical implementation of non-invasive prenatal diagnosis
for single gene disorders: challenges and progress made. Prenat Diagn.
2013 Jun;33(6):555-62.
http://dx.doi.org/10.1002/pd.4124
[7] Chitty LS, Khalil A, Barrett AN, Pajkrt E, Griffin DR, Cole T. Safer,
accurate prenatal diagnosis of thanatophoric dysplasia using ultrasound
and cell free fetal DNA. Prenat Diagn. 2013 May;33(5):416-23.
http://dx.doi.org/10.1002/pd.4066
NIHR Programme Grant: RAPID- Reliable Accurate Prenatal
non-Invasive Diagnosis RP-PG-0707-10107, sponsor GOSH
2009-2014, £2 million
Details of the impact
Guidelines and adoption
The research on non-invasive prenatal diagnosis (NIPD) contributed to a
report from the PHG Foundation in 2009, giving a service-based overview of
the implications for the NHS of implementing this technology [a].
We also produced a opinion paper on NIPD using cell free fetal DNA in
maternal blood for the Royal College of Obstetricians and Gynaecologists
(RCOG) in 2009 which was supported by their scientific advisory committee
[b]. We then led the Gene Dossier submission to the UKGTN which was
approved formally in April 2011 [c]. Furthermore from 2012 the
approval of Gene Dossiers for Achondroplasia and Thanatophoric dysplasia
was gained [d, e]. Chitty has also co-led the FP7 work package of
Eurogentest 2, which has developed and published guidelines for service
delivery in Europe [f]. The technology has attracted further
interest from policy makers, including a report on genomic technology in
healthcare by the Human Genomics Strategy Group for the Department of
Health [g].
Service provision and patient benefit
NIPD for fetal sex determination is now the recognised standard of
practice in UK genetic services allowing equity of access for all women in
the UK at high risk of sex-linked disorders. The service at Great Ormond
Street Hospital (GOSH) performs >100 tests per annum (Table 1) and,
using samples in the RAPID sample bank, has helped other laboratories
establish this as a standard of care, with Manchester offering this test
from 2010, Birmingham from 2011 and Cambridge, Edinburgh and Salisbury
from 2013. Fetal sex determination using NIPD is now the most common
prenatal molecular test performed in the UK [h] and has reduced
the invasive testing rate by nearly50% for women at high risk of
sex-linked disorders.
We have established a large unique/comprehensive bank of samples that is
a resource for academic and commercial collaborators, which has already
helped establish NIPD for sex determination in four other UK laboratories,
and is being used to develop NIPD for Duchenne Muscular Dystrophy,
Tuberous Sclerosis and Huntingdon Disease in Birmingham, Cambridge and
Edinburgh regional genetics centres, as well as developing non-invasive
prenatal testing for aneuploidy and other chromosomal rearrangements. We
are the only public service laboratory offering a clinical service for
NIPD for single gene disorders - not just in UK, but beyond, and we
receive referrals from Europe, Canada and North America (Table 1).
|
NIPD for fetal sex
determination
|
Prenatal tests for
Achondroplasia
|
Prenatal tests for
Thanatophoric dysplasia
|
|
|
|
|
Invasive
|
NIPD |
Invasive
|
NIPD
|
|
|
96 |
21 |
|
4 |
|
|
|
118 |
28 |
|
16 |
|
|
|
103 |
27 |
13 |
21 |
0 |
|
|
124 |
28 |
14 |
25 |
2 |
|
|
163 |
20 |
22 |
17 |
11 |
|
|
79 |
10 |
4 |
4 |
11 |
|
Other tests performed
clinically
|
Apert syndrome (n=7)
Osteogenesis Imperfecta (n=1)
Torsion dystonia
(n=4)
Fraser's syndrome (n=1)
Autosomal Recessive Polycystic Kidney Disease (n=1)
|
Sources of referrals
|
UK, USA, Canada, Netherlands, Italy, Norway,
Switzerland
|
Table 1. Details of clinical NIPD tests performed by our Regional
Genetics Laboratory (figures given by financial year). Note the steady
increase in numbers of tests done over time, with the trend to decreased
invasive testing following gene dossier approval in 2012 [i].
These tests can be offered earlier in pregnancy further relieving
parental anxiety. The benefits are summed up by the supporting statement
one patient gave us when we submitted our application for an NIHR
programme grant to further develop this work and has been further
supported in our work with patients who have undergone NIPD [reference 4
in section 3 above]:
"It is only three weeks since the termination, though the experience
is stil raw I wanted to share with you that the pain is very much mixed
with a great sense of gratitude for the opportunity of having early
non-invasive testing. Having experienced both procedures, I am
enormously appreciative of developments in cffDNA diagnosis. Even with
its unfortunate outcome, my second testing experience was a
significantly less distressing process than the CVS with extended
waiting period and associated risks. I would sincerely love to see the
service and support I experienced expanded as far as possible, so that
others can benefit as I did" [j].
Patient and Practitioner Engagement
Our third impact is the engagement with practitioners and
patients, particularly through our website www.rapid.nhs.uk
which provides an information resource required to implement this
safer approach to prenatal testing whilst maintaining the informed patient
consent. In partnership with the National Genetics Education and
Development Centre, Birmingham (RAPID co-applicants) and lay organisations
such as Genetic Al iance UK, Sickle Cell Association and Antenatal Results
and Choices (ARC) using information acquired from patient interviews and
surveys, we have developed health information packages, including
e-learning modules [k]. We have also contributed to
practitioner-facing journals [l].
Sources to corroborate the impact
[a] PHG foundation Steering Group Wright, C. Cell-free fetal nucleic
acids for non-invasive prenatal
diagnosis, Report of the UK expert working group, PHG Foundation (2009): http://www.phgfoundation.org/download/ffdna/ffDNA_report.pdf
[b] Chitty LS, Crolla JC. Non-invasive prenatal diagnosis using cell free
fetal DNA in maternal blood. Scientific Advisory Committee Opinion Paper
15, RCOG June 2009: http://www.rcog.org.uk/files/rcog-corp/uploaded-files/SIP_No_15.pdf
[c] Gene Dossier submission to the UKGTN which was approved formally in
April 2011 Approval:http://ukgtn.nhs.uk/find-a-test/search-by-disorder-gene/test-service/x-linked-conditions-excluding-haemophilia-nipd-602/
Best Practice guidelines:
http://ukgtn.nhs.uk/fileadmin/uploads/ukgtn/Documents/Resources/Library/NIPD/BPCAREPATWAYSNIPDCAHFINAL.pdf
[d] Approval of Gene Dossiers for Achondroplasia and Thanatophoric
dysplasia:
[e] Corroboration of our impact on the approval of the gene dossiers is
available from the Chair of UKGTN Clinical and Scientific Advisory Group.
Contact details provided.
[f] Skirton H, Goldsmith L, Jackson L, Lewis C, Chitty L. Offering
prenatal diagnostic tests:European guidelines for clinical practice
guidelines. Eur J Hum Genet.
http://doi.org/pds
[g] Building on our inheritance: Genomic technology in healthcare. A
report by the Human Genomics Strategy Group. January 2012 gives NIPD as an
example that needs to be developed (quotes and Chitty acknowledged for
contribution): http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_132382.pdf
[h] CMGS audits 2010-11 and 11-12:
www.cmgs.org/CMGS%20audit/cmgs_audit.htm
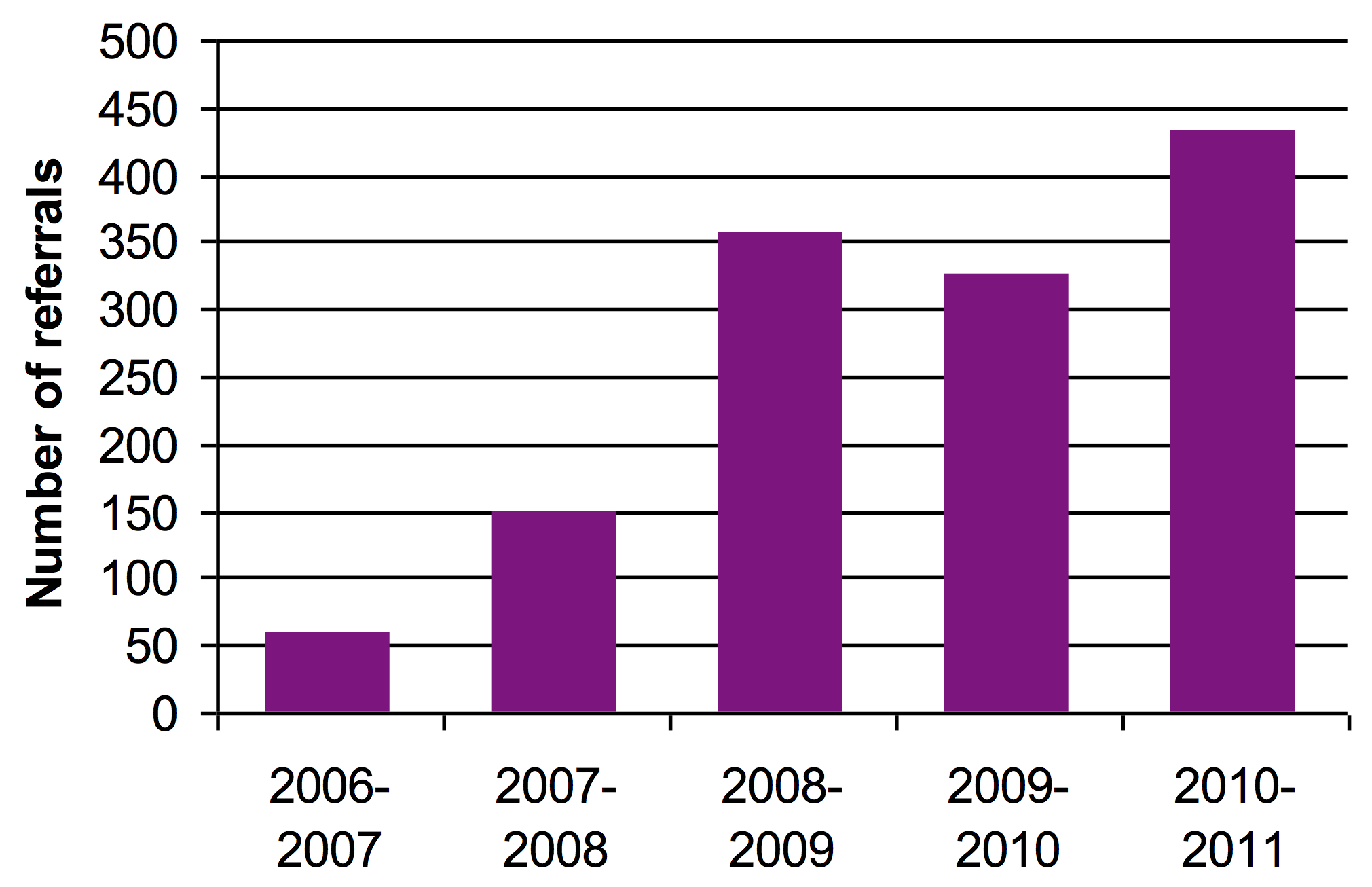
Fig 1a. Histogram showing increase use of NIPD for fetal
sex determination
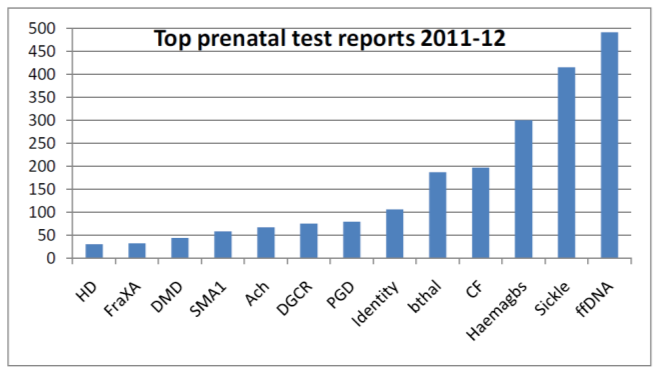
Fig 1b. Molecular prenatal tests performed
[i] Data can be confirmed by Lead Scientist NIPD section, North East
Thames Regional Genetics Service, Great Ormond Street Hospital NHS
Foundation Trust. Contact details provided.
[j] Anonymised patient feedback available on request from Great Ormond
Street Hospital.Contact details provided. Further quotes from interviewed
patients who have undergone NIPD and these are published in Lewis C, Hil
M, Skirton H, Chitty LS. Fetal sex determination using cell-free fetal
DNA: service users' experiences of and preferences for service delivery.
Prenat Diagn. 2012; 32(8):735-41 and Lewis C, Hil M, Chitty LS:
Non-invasive prenatal diagnosis for single gene disorders: experience of
patients. Clin Genet 2013. http://doi.org/pdt
[k] See www.rapid.nhs.uk. Activities
include:
-
RAPID dissemination meetings at ICH for laboratory and clinical
staff across England - July2009, January 2010, November 2011, November
2012
[l] Director, Antenatal Results and Choices can vouch for support and
dissemination to patients.Contact details provided.
[m] Rafi I, Chitty L. Cell-free fetal DNA and non-invasive prenatal
diagnosis. Br J Gen Pract. 2009 May;59(562):e146-8.
http://dx.doi.org/10.3399/bjgp09X420572.