Topical oxygen therapy for wound healing
Submitting Institution
University of CambridgeUnit of Assessment
Electrical and Electronic Engineering, Metallurgy and MaterialsSummary Impact Type
TechnologicalResearch Subject Area(s)
Chemical Sciences: Inorganic Chemistry, Macromolecular and Materials Chemistry, Physical Chemistry (incl. Structural)
Summary of the impact
A small, battery-powered device for oxygen generation and distribution (Natrox™),
has been developed that, with air as input, can supply humidified oxygen
evenly to wounds, such as ulcers, surgical wounds and burns, allowing the
patient to be treated in a discrete efficient way without interfering with
their lifestyle. With conventional approaches, oxygen can be supplied to
hospital patients with ulcers only via gas bottles or piped oxygen, with
the limb or body being enclosed in a plastic bag. Many successful trials
of the Natrox™ device have been performed, initiating considerable
interest, leading to the manufacturing and distribution of the device by InotecAMD
Ltd, a University of Cambridge spin-out.
Underpinning research
The Materials Chemistry Group, Dept of Materials Science &
Metallurgy, University of Cambridge has been very active, for many years,
in aqueous electrochemistry. In particular, Nafion membranes,
which are proton conductors under humid conditions, were used to create
hydrogen sensors [1] and membranes for electrowinning cells [2,3].
Furthermore, in work initiated in 1993, hydrophobic porous membranes were
used to create fine bubbles (sparging) in electrowinning cells
[2,3]. Critical lessons (on the benefits of smaller gas bubbles and how
they can be achieved) from this hydrometallurgy work were directly
applicable in developing the Natrox™ device for oxygen generation.
An earlier oxygen-generating device had been developed by others in the
US, but this device had several disadvantages, including: failure of the
cell due to drying out of the electrolyte; formation of hydrogen peroxide
which is a known carcinogen; and, lastly, oxygen was fed by a
small-diameter tube (cannula) to the wound (a method not favoured
by UK clinicians).
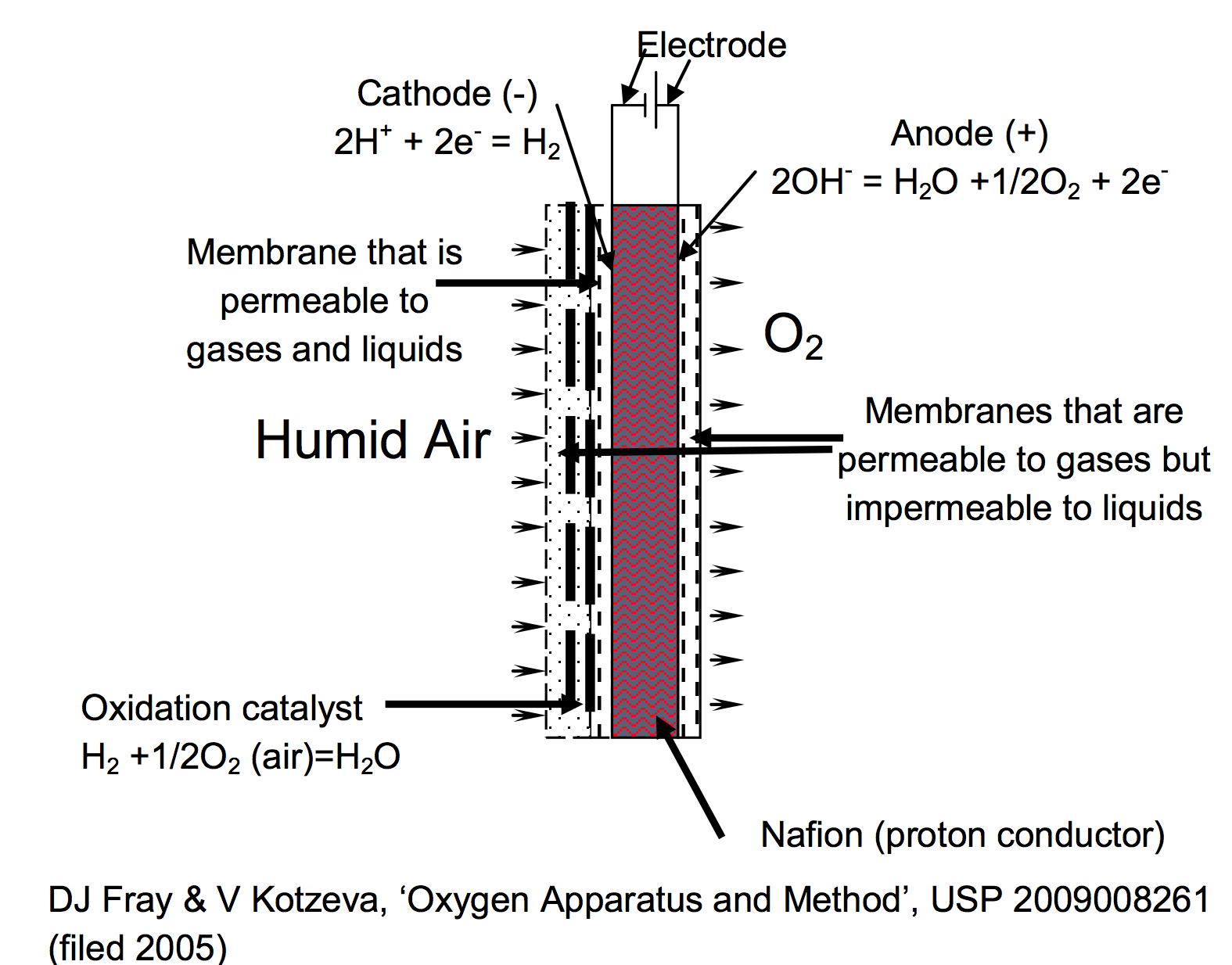 Figure 1. Diagram of Natrox™ oxygen generator showing, on the right, the electrochemical cell that produces the oxygen from water in the membrane and the oxidation catalyst, on the left, where the hydrogen is reacted with oxygen from the air, to produce water that is returned to the membrane.
Figure 1. Diagram of Natrox™ oxygen generator showing, on the right, the electrochemical cell that produces the oxygen from water in the membrane and the oxidation catalyst, on the left, where the hydrogen is reacted with oxygen from the air, to produce water that is returned to the membrane.
Building upon previous research, described in the first paragraph, Derek
Fray (Professor of Materials Chemistry 2001-2007, Director of
Research and Emeritus Professor of Materials Chemistry 2007-)
concluded that it was feasible to create a device that overcame all of
these problems. Together with Dr Vega Kotzeva, (Post Doctoral
Research Associate 2005-2006), a small cell was built, based on a design
that had been previously used for a hydrogen sensor [1], which used a Nafion
membrane surrounded by a small reservoir of water.
This successfully demonstrated that, by applying a small voltage (1.5 V)
across the membrane, a flow of oxygen was created which would satisfy the
needs of an average ulcer (15 ml/h) with a weekly consumption of water of
1 ml. It was thought desirable to oxidize the hydrogen (a by-product of
the electrolysis process) by placing a catalyst layer a short distance
from the cathode, allowing the hydrogen to react with air to form water
which then returns to the membrane [4,5]. Rather surprisingly, it was
found that with a combination of hydrophobic and hydrophilic membranes it
was possible to operate the device indefinitely, producing humidified
oxygen without the need for a water reservoir, provided the batteries were
kept charged. A diagram of the cell is shown in Figure 1. The distribution
pad for the oxygen was, again, created using the discoveries in the
hydrometallurgical studies of sparging. This previous research
showed that, by applying a modest gas pressure, a porous hydrophobic
membrane allowed the creation of a uniform supply of bubbles [2,3]. This
is ideal for supply of oxygen to a wound but, in order for the exudate to
escape, it is necessary to also have very much larger holes in the
membrane. The end result is a pad that allows oxygen bubbles to be created
at the wound and the exudate successfully removed (Figure 2 [6]).
Furthermore, unlike the cannula approach, the hydrophobic pad does not
stick to the wound which makes the removal of the pad from the wound easy
and painless. The combination of the oxygen generator and oxygen-delivery
system has been trademarked as Natrox™.
References to the research
2*. F Tailoka & DJ Fray: Enhancement of mass-transfer using
microporous sparger materials, Trans Inst Mining Metal 102
(1993) C1.
3. F Tailoka & DJ Fray: Electrowinning of copper from chloride
solutions in presence of gas sparging, Trans Inst Mining Metal 102
(1993) C7.
4*. DJ Fray & VP Kotzeva, `Oxygen Apparatus and Method'. Filed
3/03/2005. Published patents: US2009008261, WO2006092612, GB2431668*,
EP1856307, CN2068001317*
5. MF Vinton & DJ Fray, `Oxygen Concentrator and Method'. Filed
13/09/2010. Published patents: WO2102035298, GB248520*
6. MF Vinton, A Hurst & DJ Fray, `Hyperbaric Dressing'. Patent filed
1/04/2005. Published patents: US2008269658,WO2005094744, GB2412589*,
EP1755510, CN200580017913*
*references best indicating the quality of the underpinning
research.
The three patents [4-6 above] have been published and are either under
examination or granted. The distinction of Professor Fray's research in
electrochemistry has been widely recognised. During the REF assessment
period he was elected to Fellowship of the Royal Society, and he was the
first person to receive the Federation of European Materials Societies' Innovation
Award. In 2011, an International Conference (with some 450
delegates) was held in his honour in Cancun, Mexico.
Details of the impact
Professor Fray first had the ideas for exploiting the
hydrometallurgical results and adapting them for oxygen generation in 2001
(ie before the assessment period for research in the REF). It wasn't until
2003, when in discussion with Melvin Vinton, it was decided to
form a company, InotecAMD Ltd [i] (references in Section 5), to
initially design an oxygen-distribution system, funded by a Smart Award.
In 2005, the Dept of Materials Science & Metallurgy at UCAM funded a
one-year research project (£100k) to develop the oxygen-generation system.
This device was a success and was patented by the University in 2005 and,
subsequently, licensed to InotecAMD Ltd on 27 July 2009, with an
effective date of 1 January 2008. From 1 January 2008 to 31 July 2013,
[text removed for publication]
This allowed the oxygen-generation system to be industrialized and the
oxygen-distribution system to be perfected.
Natrox™ is the first device that can successfully
deliver oxygen, almost indefinitely if the batteries are kept recharged,
to patients without interfering with their lifestyle.
Over 200 Natrox™ units have been made with many being used for random
controlled trials at three hospitals in Prague. The remainder have
been used for trials in the UK and sent to agents/distributors in the UK,
USA, France, Singapore, Malaysia, Turkey and Dubai.
 Figure 2. The oxygen generator and circular pad in close-up (on the left), and positioned on the leg of a mannequin (right). The oxygen is evenly distributed over the wound by the pad and the large spaces in the pad allow the escape of exudate.
Figure 2. The oxygen generator and circular pad in close-up (on the left), and positioned on the leg of a mannequin (right). The oxygen is evenly distributed over the wound by the pad and the large spaces in the pad allow the escape of exudate.
Health impacts
To check the viability of the Natrox™ device, trials were
performed at the end of 2009 at a Tissue Viability Clinic in Eastbourne,
which dramatically showed the efficacy of such a device with 10 patients
who had been referred to the clinic by the NHS as not treatable; 8 showed
considerable improvement after six weeks treatment [iv]. A bonus not
originally considered was that the pain, experienced by the patients, also
decreased considerably when oxygen was fed to their wounds [iv]. Further
successful trials have been carried out at Doncaster Royal Infirmary
on hard-to-heal wounds as the result of surgery. An evaluation of oxygen
therapy was undertaken on a range of complex surgical wounds, which
included post-mastectomy wounds which had dehisced (ie opened) following
surgery. It was found that, after about six weeks treatment with oxygen,
the wounds improved dramatically and conventional treatment could then be
applied to procure a complete recovery. It was concluded that this therapy
was successful, both in promoting healing and improving the quality of
life in a group of anxious patients [v]. In early 2012, the Natrox™
system was subjected to a safety study at the FNKV Hospital in Prague on
ten patients with non-healing leg ulcers and all patients showed
considerable shrinkage of their ulcers.
In June 2012, InotecAMD Ltd received a CE mark
for the oxygen-delivery system giving all the regulatory approvals for
sales in the EU. In July 2012, the US FDA awarded the 510(k) approval
for the Natrox™ system meaning that Natrox™
can be sold in the US [vi].
At present, a random controlled trial is being performed in Prague on ~60
patients so that the treatment can be sold to the NHS. In November 2013,
Doncaster & Bassetlaw hospitals will embark on a 12-month trial
studying non-healing surgical wounds and breast reconstruction [v].
Overall, about 80 patients have been successfully treated, ie healed,
with a considerable decrease in their suffering, coupled with an
increase in their general wellbeing.
Impacts on public policy and services
About 2 million patients in the European Union suffer from non-healing
ulcers, and the annual cost of treating these is estimated to be 10
billion Euros. The clinical trials provide hard evidence that the use of Natrox™
would decrease the number of patients and shorten treatment times. Natrox™
has opened up possibilities for more cost-effective healthcare, with
better outcomes. Secondly, the rationale for the trials at Doncaster &
Bassetlaw hospitals is to study oxygen as an anti-microbial treatment, as
many patients have increased resistance to antibiotics [v]. The use of
oxygen instead of antibiotics for treating infected wounds would again
improve both outcomes and the cost-effectiveness of the service.
Economic impacts
As well as cost savings to national budgets, the manufacture and supply
of Natrox™ devices will generate employment and income for the UK.
In 2013, the company employed a part-time CEO, a full-time Chief Technical
Officer and three other part-time staff. In addition, much of the
equipment, circuitry, oxygen-distribution pad and units are manufactured,
under subcontract, in the UK. The random controlled trial is being
organized by UK-based SME Wound Market Consulting
(www.woundmarketconsulting.com). Sales of Natrox™ devices have
commenced in the Far East, and distributors in UK and France are
organizing trials, prior to sales. Virtually all the investment of [text
removed for publication]
increase the rate of production, to
investigate a wider range of applications for the device such as treatment
of other types of ulcers, burns and surgical wounds, and to employ more
technical and sales personnel.
Sources to corroborate the impact
[i] InotecAMD Ltd (Chairman, Chief Executive Officer, Chief
Technical Officer) (www.inotecAMD.com)
— for corroboration of all information on the development of the Natrox™
device, current tests, approvals, marketing, orders, etc.
[ii] 2005 http://www.angelnews.co.uk/article.jsf?articleId=1158
[iii] 2009 http://www.praxisunico.org.uk/news/member-detail.asp?ItemID=254
[iv] R Mani: Topical oxygen therapy for chronic wounds: a report on the
potential of Inotec® a new device for delivering enriched oxygen to
chronic wounds, J. Wound Technology, no. 9 (July 2010)
1-4.
[v] Doncaster and Bassetlaw Hospitals NHS Foundation Trust: (Sister,
Wound Care Service) — for corroboration of effectiveness of the Natrox™
device for wound care, particularly after mastectomy.
[vi] 2012 FDA approval of Natrox™ —
http://www.accessdata.fda.gov/cdrh_docs/pdf11/K112634.pdf