Three spin-out companies built on platform Lab-on-a-chip technologies deliver diagnostic tools for infection and disease
Submitting Institution
University of GlasgowUnit of Assessment
General EngineeringSummary Impact Type
TechnologicalResearch Subject Area(s)
Physical Sciences: Other Physical Sciences
Chemical Sciences: Analytical Chemistry
Summary of the impact
Fifteen years of research in advanced Lab-on-a-Chip technologies at the
University of Glasgow has led to three spin-out companies: Mode-Dx, Clyde
Biosciences and SAW-Dx. Since 2008 these companies have developed a range
of products and services for the diagnostic screening of chronic diseases,
for the detection of acute infections and for improving the drug discovery
process. The three companies have secured a total of £2.3M in venture
funding and secured key strategic collaborations with stakeholders
including industry partners and the NHS.
Underpinning research
Prof Jonathan Cooper (Lecturer 1991-96, Senior Lecturer 1996-98,
Professor 1998-present) leads the Advanced Medical Diagnostics Group in
the School of Engineering, and has developed new Lab-on-a-Chip and
biosensors technologies, based upon acoustic, optical and electrochemical
systems.
Lab-on-a-Chip research in Cooper's group started in 1999, supported by a
£3.2M DTI-EPSRC Foresight LINK "Lab on a Chip" programme. The project was
co-ordinated by Dr Derek Craston (LGC, now also Government Chemist), and
was carried out in collaboration with GlaxoSmithKline (GSK), Unilever and
Kodak together with a group of SMEs, including Epigem. Cooper's research
has enabled the development of new, advanced microfabrication techniques
for on-chip sensing and interconnect technologies for microfludics.
Cooper, Dr Igata (Research Assistant (RA) 2000-01) and Dr Arundell (RA
2002) integrated analytical technologies into microfluidic devices using
novel fluidic handling strategies for both diagnostic and cell based
assays [1].
This work was followed by a Scottish Higher Education Funding Council
grant, `Integrated Diagnostics for Environmental and Analytical Systems'.
This supported underpinning research in which Cooper and Dr Johannessen
(RA, 2001-04) developed sensors and optimised packaging to create a fully
functional ingestible prototype diagnostic device (or pill). This work,
when combined with the earlier DTI-EPSRC research, was instrumental in
producing a pill-based sensor [2], a concept which led to the formation of
Mode-Dx.
In parallel with these activities, the EPSRC supported the
Bio-Nanotechnology Interdisciplinary Research Centre (IRC), a
collaboration with Professor Ryan (University of Oxford) and Dr Molloy
(National Institute for Medical Research) (GR/R45659/01, 2002-09). Here,
Cooper led the cellular nanotechnology theme that developed tools for
exploring cell-based assays in microfluidic systems. This research, which
was carried out in collaboration with Professor Smith (Institute of
Cardiovascular and Medical Sciences, University of Glasgow, 1997-present),
and Dr Klauke (RA 1999-2009, Research Technologist 2009-11), developed
microarrays for electrically stimulating heart cells [3].
Working within a DTI-funded Micro and Nano Technology Programme in
2005-08 with Dr Warrington and Dr Cordingley (GSK), Dr Ryan (Epigem), and
Dr Craston (LGC), the University of Glasgow group translated their work
into an industrial context and developed high-throughput microfluidic
cell-based assays for pharmaceutical testing (relating the new platform to
industry-standard assays [4]). Supported by BBSRC (BB/H013369/1) and a
Scottish Enterprise Proof-of-Concept Grant (POC/BPT011), Cooper and Smith
subsequently implemented these high-throughput technologies as new
fluorescence based assay formats to explore cardiotoxic effects of drugs
on heart cells. A prototype instrument for optical measurements of action
potential and ion flux was developed with Martyn Reynolds at Cairn
Research, which resulted in the formation of Clyde Biosciences in 2012 to
provide low-cost, early assessment of cardiotoxicity as part of the
development process of new medicines.
Most recently, research within the field of acoustics and microfluidics
which was underpinned by long-term IRC funding in Proteomic Technologies
(BB/C511572) and a Research Landscape Grant (EP/I017887/1) enabled Cooper,
Dr Wilson (RA 2005-present) and Dr Reboud (RA 2009-12, Research Fellow
2012-present) to develop frequency dependent phononic structures for
shaping ultrasonic fields [5]. This invention is described in
WO/2012/114076, whilst methods for manufacturing the technology, developed
with Dr Ryan of Epigem under a TSB grant (TSB/61-135, 2010-12), are
described in WO/2011/023949. Work within a Scottish Enterprise
Proof-of-Concept grant (POC/13-LSM003) permitted the microfluidic research
to be translated into a prototype instrument for DNA testing of infectious
diseases, and led to the formation of SAW-Dx. Most recently, the
technology was validated through the detection of malaria in blood [6],
work supported by the Bill and Melinda Gates Foundation (Grant
OPP1032927).
References to the research
1. Igata, E., Arundell, M, Morgan, H, and Cooper, J.M. (2002);
Interconnected Reversible Lab-on-a-Chip Technology. Lab on a Chip, 2, pp.
65-69. ISSN 1473-0197 (doi: 10.1039/b200928p).
2. Johannessen, E.A., Wang, L., Reid, S.W.J., Cumming, D.R.S., and
Cooper, J.M. (2006); Implementation of Radiotelemetry in a Lab-in-a-Pill
Format, Lab on a Chip, 6, pp. 39-45. ISSN 1473-0197 (doi: 10.1039/b507312j).
3. Klauke, N., Smith, G.L. and Cooper, J.M., (2003); Stimulation of
Single Isolated Adult Ventricular Myocytes within a Low Volume using a
Planar Microelectrode Array, Biophysical Journal, 85, pp.1766-1774, ISSN
0006-3495, (doi: 10.1016/S0006-3495(03)74606-2).
4. Yin, H., Pattrick, N., Zhang, X.L., Klauke, N., Cordingley, H.C.,
Haswell, S.J., and Cooper, J.M. (2008); Quantitative Comparison between
Microfluidic and Microtiter Plate Formats for Cell-Based Assays,
Analytical Chemistry, 80, pp.179-185, ISSN 0003-2700 (doi: 10.1021/ac701958z).
*
5. Wilson, R., Reboud, J., Bourquin, Y., Neale, S.L., Zhang, Y. and
Cooper, J.M., (2011); Phononic Crystal Structures for Acoustically Driven
Microfluidic Manipulations, Lab-on-a-Chip, 11, pp. 323-328 (doi: 10.1039/C0LC00234H).
*
6. Reboud, J., Bourquin, Y., Wilson, R., Pall, G.S., Jiwaji, M., Pitt,
A.R., Graham, A., Waters, A. and Cooper, J.M. (2012); Shaping Acoustic
Fields as a Toolset for Microfluidic Manipulations in Diagnostic
Technologies, Proceedings of the National Academy of Sciences, 109, pp.
15162-15167, ISSN 0027-8424, (doi: 10.1073/pnas.1206055109).
*
* best indicators of research quality
Details of the impact
Cooper's research in medical diagnostics is driven by the demand for new
miniaturised formats providing low-cost, disposable point-of-care devices,
with applications in near-patient/bathroom environments for screening
chronic diseases and diagnosing acute microbial infections. Similarly, the
new miniaturised formats of Cooper's work have been applied to the drug
discovery process within the pharmaceutical industry through the
development of tools that have improved the ease of data acquisition.
These tools replace labour-intensive patch-clamp electrophysiological
measurements with high-throughput measurements which enable toxicological
information on candidate drugs to be obtained earlier in the development
cycle. Thus, Cooper's research has generated impact through three
spin-out companies providing advanced measurement technologies for a
range of applications:
Mode-Dx (http://www.modedx.com):
Mode Diagnostics (Mode-Dx) was launched in 2008 as a company developing
digital homecare diagnostics. It now employs eight staff and in 2009
secured £1.7M of investment from the IP Group, the Scottish Investment
Bank and the syndicated investor Kelvin Capital. It received a £72k SMART
award in 2011, together with a £23k Innovation Award from Scottish
Enterprise in 2011.
Mode-Dx has developed low-cost, easy-to-use electrochemical diagnostic
products for the consumer market. Following this successful development
work, the company attracted John Brown, formerly Chairman of Axis-Shield,
as its Chairman in 2012. The first product, a colorectal cancer
diagnostic, called measure® BOWEL HEALTH, Figure 1, detects occult
haemoglobin as a proxy for bowel cancer. This product, which is focused on
physician-led screening, has been developed under full ISO processes and
is now CE-marked, with a full product launch due in 2014. Mode-Dx is also
in advanced discussions with a major UK retail pharmacy chain over the
placement of this product throughout the UK for over-the-counter home use
applications.

 Figure 1: ModeDx, Left, measure® BOWEL HEALTH is a hand-held biosensor for measuring faecal occult blood; Right, schematic showing an exploded view of the faecal sampling interface to the biosensor device.
Figure 1: ModeDx, Left, measure® BOWEL HEALTH is a hand-held biosensor for measuring faecal occult blood; Right, schematic showing an exploded view of the faecal sampling interface to the biosensor device.
Clyde Biosciences (http://www.clydebiosciences.com):
Cardiotoxicity is a major cause of failure of new medicines in the
pharmaceutical development process. Clyde Biosciences produces
instrumentation, services and biological products for both pharmaceutical
companies and contract research organisations to identify these toxic
effects earlier in the development process, thereby reducing costs. The
technology was configured into a new, high-throughput, low-cost instrument
(Figure 2, on left) in collaboration with Cairn Research. This led to Dr
Margaret-Ann Craig being awarded the prestigious Royal Academy of
Engineering ERA Foundation Entrepreneurship Award (2012) for developing
new optical instrumentation, bespoke software (Figure 2, on right) and
microsystems technologies for evaluating new drugs. Subsequently
seed-funding (£50k in 2012) secured through the University's partnership
with IP Group has enabled the successful development of a business model
and strategic industrial collaborations, as well as the sale of
instruments. The company also received a SMART Award (Project value £137k)
in 2013.
Clyde Biosciences has now launched three products: CellOPTIQ (an optical
instrument to assess the toxicology of new medicines); XTEND(SR) (for
direct measurements of drug-induced changes on cardiac cells); and XTEND
(which extends the life of tissue samples). Clyde Biosciences is in the
process of selling its first two cell screening systems (total £330k) to
Imperial College and INSERM and has
developed two partnerships with Astra Zeneca and Johnson & Johnson
(both involving contracts for screening of drug candidate libraries).

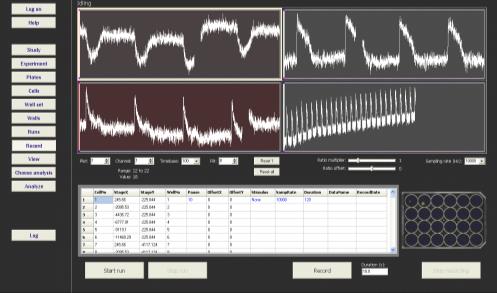 Figure 2: Clyde Biosciences (left) the CellOPTIQ instrument; (right) proprietary image-analysis software enabling real-time, multiplexed electrophysiological measurements.
Figure 2: Clyde Biosciences (left) the CellOPTIQ instrument; (right) proprietary image-analysis software enabling real-time, multiplexed electrophysiological measurements.
SAW-Dx: SAW-Dx is a micro-SME, which uses phononic crystals as
`acoustic holograms' to control the interaction between the ultrasonic
fields generated using surface acoustic wave (SAW) devices and the
diagnostic sample. Funding from the Scottish Enterprise Proof-of-Concept
Fund (2010-12) enabled the technology to be implemented on low-cost
disposable chips, coupled into the ultrasonic piezoelectric transducers. A
manufacturing technology has been developed with Epigem through a
TSB-funded programme (TS/1000097/1, 2010-12). Dr Reboud was awarded the
Royal Academy of Engineering ERA Foundation Entrepreneurship Award (2013)
to translate the technology into a DNA-based diagnostics technology,
leading to seed venture funding from IP Group in 2013. The company was
formed in March 2013 and now works on three products.
Firstly, in a development programme with a consultant in Sexual Health
& HIV Medicine, NHS Greater Glasgow and Clyde and funded by the NHS,
SAW-Dx is developing integrated diagnostics for sexual health. The product
uses the company's proprietary technologies for sample preparation and
rapid detection, including a new acoustically driven, multiplexed DNA
isothermal amplification protocol. The aim is to break the cycle of
infection and treatment, by providing rapid diagnosis of a panel of
pathogens, enabling the patient to be treated prior to leaving the clinic.
A second product, focussed on food security, has seen SAW-Dx adapt its
multiplexed human DNA technologies for sexual health to veterinary
applications, with a specific focus on disease diagnosis in cattle and
buffalo artificial insemination stations in India. India produces 125bn
litres of milk per annum and the `white revolution' of cow and buffalo
milk production underpins its economy. SAW-Dx works on developing these
products with the Indian Veterinary Research Institute and the UK Animal
Health and Veterinary Laboratory Agency.
Finally, SAW-Dx has also secured a TSB grant (TS/L003392/1), working with
MV Diagnostics Ltd and Epigem to develop DNA and protein biomarker tests
for tuberculosis (TB), and sees longer-term products based upon developing
assays for rapid TB testing.
Sources to corroborate the impact
- Statement from and contact details for CEO, Mode-Dx (role of the
University in the formation of Mode-Dx)
- Statement from and contact details for CEO, Clyde Biosciences (CB) (on
role of the University in the formation of CB)
- IPG (on Venture funder and Shareholder of Mode-Dx, Clyde Biosciences
and SAW-Dx) (corroborating the role of IPG as a Venture funder and
Shareholder of Mode-Dx, Clyde Biosciences and SAW-Dx)
- Director, Bowel and Cancer Research (on importance of Mode-Dx
technology as a home diagnostic test) (contact details provided)
- CEO, Epigem Limited (developing manufacturing technologies and
relationship with SAW-Dx)
- Article on Clyde Biosciences (link)
(contact details provided)