Cochrane Oral Health Group leads the international evidence base for oral health: Antibiotics for the prevention of bacterial endocarditis. (ICS-07)
Submitting Institution
University of ManchesterUnit of Assessment
Allied Health Professions, Dentistry, Nursing and PharmacySummary Impact Type
HealthResearch Subject Area(s)
Medical and Health Sciences: Public Health and Health Services
Summary of the impact
Life-threatening bacterial endocarditis occurs on previously damaged
cardiac valves. Established
dental practice has been to administer antibiotics to patients who are at
risk. This practice has
been linked with increased antibiotic resistance, which represents one of
the greatest threats to
public health.
Researchers at the University of Manchester (UoM) evaluated the evidence
for this practice by
undertaking a high quality systematic review (initially published 2004).
The review has informed
multiple international guidelines. Publication of the NICE guideline led
to a fall in the unnecessary
prescription of antibiotics from 10,727 to 2,292 per month, an approximate
annual saving of
£174,580.
Underpinning research
See section 3 for references [1-2]; see section 5 for corroborating
sources (S1-S10); UoM
researchers are given in bold. In REF3a and REF5 this case study is
referred to as ICS-07.
The impact here flows from a Cochrane review, and its subsequent update,
conducted and co-
ordinated at the editorial base of the Cochrane Oral Health Review Group,
UoM. The Cochrane
Oral Health Group has been based in Manchester since 1997 and has received
consistent funding
of over £3m from the Department of Health and currently has funding until
2015. The review was
used to inform the NICE guidance issued in 2008 and received specific
funds from the Cochrane
Incentive Scheme.
Key researchers were:
-
Lee Hooper (Lecturer, 2000-2004)
-
Richard Oliver (Lecturer, 1998-2005; Senior Lecturer,
2005-2006; Honorary Consultant,
2006-2010)
-
Helen Worthington (Reader, 1998-2003; Professor of Evidence
Based Care, 2003-date)
The underpinning research was undertaken by a team of UoM content experts
and methodologists
who ensured that this review was of high quality. Randomised controlled
trials are difficult in this
area due to the low incidence of bacterial endocarditis; therefore other
study designs were also
considered, requiring new methodological approaches and specific
expertise. The review was also
supported by a team of international clinical experts.
- We undertook a systematic review which initially investigated
penicillin for the prevention of
bacterial endocarditis in patients having dental treatment [1].
- Authors' conclusions from 2004 review: there is no evidence about
whether penicillin
prophylaxis is effective or ineffective against bacterial endocarditis
in people at risk who are
about to undergo an invasive dental procedure [1].
- We undertook an update of the review, extending it to all antibiotics;
the conclusions
remained the same [2].
Based on the reviews conducted it was concluded that there is a lack of
evidence to support
published guidelines which underpin the prescribing of antibiotics
prophylaxis for dental procedures
to prevent bacterial endocarditis. There are potential harms and costs of
antibiotic administration
that outweigh any beneficial effect. There is also an ethical need for
practitioners to discuss the
potential benefits and harms of antibiotic prophylaxis with their patients
before a decision is made.
This review continues to be one of the COHG's priority reviews which is
closely monitored with
regard to the need for updating.
References to the research
The review was published in The Cochrane Library.
1. Oliver R, Roberts GJ, Hooper L. Penicillins for
the prophylaxis of bacterial endocarditis in
dentistry. Cochrane Database of Systematic Reviews 2004, Issue 2. Art.
No.: CD003813.
DOI: 10.1002/14651858.CD003813.pub2.
2. Oliver R, Roberts GJ, Hooper L, Worthington
HV. Antibiotics for the prophylaxis of
bacterial endocarditis in dentistry. Cochrane Database of Systematic
Reviews 2008, Issue
4. Art. No.: CD003813. DOI: 10.1002/14651858.CD003813.pub3.
Details of the impact
See section 5 for numbered corroborating sources (S1-S10).
Context
Many authorities have questioned the routine use of antibiotics for
endocarditis prophylaxis,
arguing that the adverse effects of antibiotics may outweigh the potential
benefits [2] (S3). The
over prescription of antibiotics by the whole medical and veterinary
professions has resulted in the
emergence of resistance of many organisms to the traditional therapeutic
antibiotics available.
Such antibiotic-resistant bacteria pose a real threat to global health,
and England's Chief Medical
Officer has called for urgent action to address the overuse of
antibiotics. The Department of
Health launched a five-year action plan in 2013 to try to address the
issue of antibiotic resistance
and ensure that they are only prescribed where truly needed. This builds
on their original 2000
strategy.
Pathway to impact
The Cochrane review sparked much international debate (S2-4) around the
prescribing of antibiotic
prophylaxis for the prevention of bacterial endocarditis, with some
dentists unwilling to change
practice, concerned about the possibly of putting their patients at risk.
Following the publication of
the NICE guidance (2008) (S1), in which the initial review [1] was used as
the highest level of
evidence (S3), the findings of the review were able to have a significant
impact which resulted in a
dramatic reduction of antibiotic prophylaxis for bacterial endocarditis.
Two authors on the review
(Oliver and Roberts) were members of the guidance development group
in the development of the
NICE guidelines. The 2008 review update [2] was produced along side the
NICE guidance (S3)
and the Cochrane Oral Health Group were stakeholders in the development of
this guidance.
Additionally, the review was used to inform other international
guidelines, including the American
Heart Association (2007) and the British Society for Antimicrobial
Chemotherapy 2006) (S5-9).
Within the UK, NICE recommendations were to stop routinely prescribing
antibiotic prophylaxis for
patients at risk of bacterial endocarditis undergoing dental and a wide
range of other invasive
procedures. An update review was undertaken to reflect emerging evidence;
no amendments to
the initial conclusions were made.
Reach and significance of impact
- The NICE guidelines which utilised the Cochrane review have
dramatically changed
practice as can be seen in the 78.6% drop in prescribing rates for
antibiotic prophylaxis in
England (Figure 1) (S 7). There has been no evidence of a large increase
in the incidence
of cases of, nor deaths from, infective endocarditis in the two years
after the guideline.
- Patients in the UK no longer routinely receive antibiotic prophylaxis
for endocarditis.
Resistance to antibiotics is one of the greatest threats to public
health. The unnecessary
prescribing of antibiotics can lead to increased resistance, which is of
concern to the
general population as a whole, not just those at risk of bacterial
endocarditis.
- Since 2008 there has been a reduction, on average, of 8,000
prescriptions of antibiotic
prophylaxis per month in England alone, resulting in a significant cost
saving of up to
£219,000 per year (S10).
- The range of potential side effects from the administration of
antibiotics is vast, largely with
a hypersensitive aetiology but some direct toxic effects may also occur.
All four types of
hypersensitivity reaction have been reported with the use of penicillins
including the most
severe reaction, anaphylactic shock, and other type I reactions
including allergic bronchial
obstruction, allergic rhinitis and angio-oedema; haemolytic anaemia,
type II, has been
recorded; drug fever, a type III reaction and the delayed type
hypersensitivity (type IV) of
allergic dermatitis. Reduction in use of antibiotics per se
reduces the risk of such adverse
reactions amongst patients.
- The review has additionally been used to inform international guidance
in the U.S.A.,
Europe and Asia Pacific (S7-9).
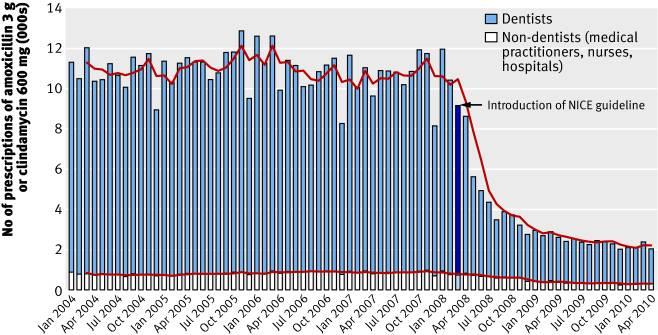 Figure 1. Total number of prescriptions for antibiotic prophylaxis
(amoxicillin 3 g or clindamycin 600 mg)
dispensed each month by type of prescriber (Thornhill et al, 2011) (S10).
Figure 1. Total number of prescriptions for antibiotic prophylaxis
(amoxicillin 3 g or clindamycin 600 mg)
dispensed each month by type of prescriber (Thornhill et al, 2011) (S10).
Sources to corroborate the impact
S1 National Institute for Health and Clinical Excellence (Wray D,
Keenan D, Franklin D,
Gibbs J, Sandoe J, Orr K, et al.) NICE clinical guideline 64:
Antimicrobial prophylaxis
against infective endocarditis in adults and children undergoing
interventional procedures
(March 2008) http://www.nice.org.uk/nicemedia/pdf/CG64NICEguidance.pdf
S2 Friedlander AH. Antibiotic Prophylaxis. Journal of the
American Dental Association
2009;140;11;1347-8
S3 Gopalakrishnan PP. Shukla SK. Tak T. Infective Endocarditis:
Rationale for Revised
Guidelines for Antibiotic Prophylaxis Clinical Medicine and Research
2009;7;3;63-8
S4 Duval X. Leport, C. Prophylaxis of infective endocarditis:
current tendencies, continuing
controversies Lancet Infectious Diseases 2008;8;225-32
S5 The Royal College of Surgeons of England and British Society for
Antimicrobial
Chemotherapy (Gould FK, Elliott TSJ, Foweraker J, Fulford M, Perry
JD, Roberts GJ, et
al.) British Society for Antimicrobial Chemotherapy Guidelines for the
Prevention of
Endocarditis (February 2006)
http://www.rcseng.ac.uk/fds/Documents/Patient%20Information%20Sheet.doc
S6 British Society for Antimicrobial Chemotherapy (Gould FK,
Elliott TSJ, Foweraker J,
Fulford M, Perry JD, Roberts GJ, et al.) Guidelines for the prevention of
endocarditis: report
of the Working Party of the British Society for Antimicrobial
Chemotherapy. Journal of
Antimicrobial Chemotherapy. 2006, 57(6): 1035-42
http://jac.oxfordjournals.org/content/57/6/1035.full.pdf
S7 American Heart Association (Wilson W, Taubert KA, Gewitz M,
Lockhart PB, Baddour
LM, Levison M, et al.) Prevention of infective endocarditis: guidelines
from the American
Heart Association Journal of the American Dental Association. June
2007, 138(6): 739-60
http://jada.ada.org/content/138/6/739.full.pdf,
and Journal of the American Dental
Association. January 2008, 139(Suppl 1): 3S-24S http://www.jada-plus.com/content/139/suppl_1/3S.full.pdf
S8 National Heart Foundation of New Zealand (Ellis-Pegler R,
Sharpe N, Everts R,
Chambers S, Hornung T, Hay KD et al.) Guideline for Prevention of
Infective Endocarditis
Associated with Dental and Other Medical Interventions (December 2008)
http://www.ttophs.govt.nz/vdb/document/312
S9 European Society of Clinical Microbiology and Infectious Diseases
(ESCMID) and by
the International Society of Chemotherapy (ISC) for Infection and Cancer
(Habib G,
Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, et al.) Guidelines
on the
prevention, diagnosis, and treatment of infective endocarditis (new
version 2009).
European Heart Journal. 2009, 30(19): 2369-413 http://www.escardio.org/guidelines-surveys/esc-guidelines/GuidelinesDocuments/guidelines-IE-FT.pdf
S10 Thornhill MH, Dayer MJ, Forde JM, Corey GR, Chu VH, Couper DJ,
Lockhart PB.
Impact of the NICE guideline recommending cessation of antibiotic
prophylaxis for
prevention of infective endocarditis: before and after study. BMJ
2011;342:d2392
http://www.bmj.com/content/342/bmj.d2392