Meeting the diagnostic needs in resource-limited settings
Submitting Institution
University of CambridgeUnit of Assessment
Clinical MedicineSummary Impact Type
TechnologicalResearch Subject Area(s)
Biological Sciences: Genetics
Technology: Medical Biotechnology
Medical and Health Sciences: Medical Microbiology
Summary of the impact
Communicable diseases are a major health burden in the developing world.
Early detection and
accurate identification of infectious agents is key to their management.
However, the complex
procedures and logistics of current diagnostic tests often make them
unsuitable for use in
developing countries. Two technology platforms have been developed that
have led to a new
generation of simple and inexpensive rapid tests that can be applied in
resource-limited settings. A
spinout company was set up to allow translation of these platforms into
new products. Three tests
(Chlamydia, Hepatitis B and HIV) were launched since 2008, with test kits
marketed, allowing
patients to receive treatment for infections which would have previously
gone unnoticed and
untreated. The spinout company has raised >$30 million, of which
>$20million is since 2008.
Underpinning research
The Diagnostics Development Unit (DDU) was established in 1996 by Dr
Helen Lee, Reader in
Medical Biotechnology, Dept of Haematology (since 1991), who left the
diagnostic industry with the
goal of developing simple, high-performance, robust yet affordable assays
for resource-limited
settings. A multi-disciplinary team was assembled to develop the
diagnostic technologies and
translate them into products. Key researchers, all in the Dept of
Haematology include C Wisniewski
(Senior Res Associate, engineering, 01/09/2007 to present), M Dineva
(Senior Res Associate, nucleic
acid chemistry, 01/10/2004 - 30/09/2010), A Ritchie (Res Associate,
molecular biology, 11/09/2006 to
present ), C Michel (Res Assistant, pilot plant manufacturing, 02/02/1998
- 31/08/2011), N Goel (Res
Assistant, quality control and probe synthesis, 23/04/2009 to present).
Two generic platform technologies that greatly improve the performance of
rapid tests have since
been developed: the Signal Amplification System (SAS)
for protein-based targets (Lin et al, JCM
2008), and Simple AMplification Based Assay
(SAMBA) for nucleic acid-based targets (Lee et al,
JID 2010).
Platform 1 - SAS, the protein-based point-of-care platform (1996 -
2008)
Current membrane-based lateral flow tests are rapid because no complex
reagent additions,
washing or incubation steps are required. However, the ease-of-use and
short assay time are
achieved at the expense of sensitivity. Whereas antibody-based rapid tests
tend to have
equivalent sensitivity to the more complex and machine-dependent Enzyme
Immunoassays (EIA),
rapid tests for the detection of antigen usually suffer from lower
sensitivity. This limitation is
particularly evident in infectious disease diagnosis for which high
sensitivity is required and yet a
low analyte level may be present.
Research in the DDU resulted in the
development of SAS, which is based on
multiplying a visual signal via an increase in
Format
the valency and size of the coloured
immune complex in the assay, by
chemically coupling multiple copies of a
hapten moiety to the primary detection
antibodies. The resulting lattice formed
between the analyte, multiple hapten-
labelled antibody and the anti-hapten colour
conjugate yields a strong visual signal. This
increases the valency and the size of
immune complexes and thereby greatly increases the visual sensitivity of
lateral flow-based rapid
tests (Fig 1).
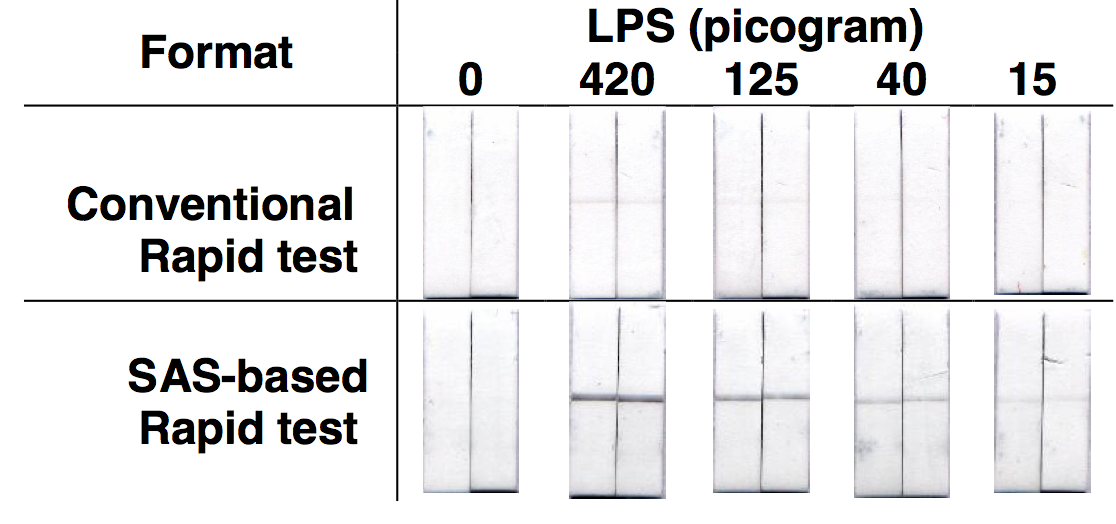 Fig 1 Effect of SAS on sensitivity of Chlamydia lipopolysaccharide (LPS) detection
Fig 1 Effect of SAS on sensitivity of Chlamydia lipopolysaccharide (LPS) detection
The sensitivity improvement of SAS for the detection of the Chlamydial
lipopolysaccharide was
used to develop a simple Chlamydia rapid test with sufficient sensitivity
that enabled the use, for
the first time of non-invasive samples such as self-collected vaginal swab
from women and urine
from men. To further improve the sensitivity of the Chlamydia Rapid test
for testing male urine, a
unique and innovative device, FirstBurstf0e4, was developed by DDU for
convenient collection of the
initial fraction of the urine stream containing 84% of the bacterial load
(Wisniewski et al, JCM
2008). SAS was also the underpinning technology that allowed the
development of a hepatitis B
surface antigen (HBsAg) rapid test which became the first rapid test to
receive the CE mark
because it was able to meet the stringent sensitivity requirement (Lin et
al, JCM 2008).
Platform 2 - SAMBA, the nucleic acid-based point-of-care platform
(2002-2008)
Existing nucleic acid tests are expensive, complex and time-consuming,
requiring sophisticated
instrumentation and highly-trained personnel. Thus, even in developed
countries, only a small
minority of clinical laboratories are capable of performing nucleic acid
amplification tests (NAT's).
The lack of simple and inexpensive nucleic acid extraction methods from
complex biological
samples with high efficiency is an important bottleneck for the
application of NAT's in resource-
limited settings. To address this critical issue, DDU developed SAMBA: a
point-of-care molecular
diagnostics platform (Fig 2) which uses novel chemistry to enable the
visual detection of nucleic
acid hybridisation at a sensitivity and specificity equal to that of
complex methods, underpinned by
a robust and simplified isothermal amplification process. The SAMBA sample
preparation protocol
takes <25 min, without requiring alcohol or chaotropic salts, and the
cartridge provides ready-made
unit dose reagents (Lee et al, JID 2010). Key
advantages of the SAMBA system include simplicity,
low technical complexity and the use of freeze-dried
reagents that are extremely stable in high temperature
and high humidity, thus circumventing the need for cold
chain storage or transport. This simplification of
complex chemistry without the need of expensive
instrumentation or highly-trained personnel is essential
to moving nucleic acid testing beyond sophisticated
clinical laboratories to the point-of-care environment in
resource-limited settings (Wu et al, JCM 2010).
 Fig 2: Simplifying NAT with SAMBA
Fig 2: Simplifying NAT with SAMBA
References to the research
A. Publications
2. Y-H. Lin, Y. Wang, A. Loua et al. Evaluation of a new sensitive
HBsAg rapid test with improved
sensitivity. J. Clin. Microbiol. 2008. 46: 3319-3324.
3. H.H. Lee, M.A. Dineva, Y.L. Chua et al. Simple
amplification-based assay: a nucleic acid-based
point-of-care platform for HIV-1 testing. J Infect Dis. 2010. 201:
Suppl 1:S65-72.
4. L.T. Wu, M.D. Curran, J. Ellis, S. et al. 2010. Nucleic acid
dipstick test for molecular diagnosis
of pandemic H1N1. J. Clin. Microbiol. 2010. 48(10): 3608-3613.
B. Intellectual properties: Eight patents have been issued in
territories including Australia,
France, Germany, India, Italy Spain, UK and USA and include:
1. Improved capture and detection format: versatility for Dipstick Assays
(PCT/GB01/03030).
GB0016833.6,7 Jul 2000. Granted: EU Validating: FR, DE, IN, UK,
USA
2. Signal enhancement system with multiple labeled-moieties
(PCT/GB01/05325). GB0029154.2,
30 Nov 2000 Granted: CN, FR, DE, IT, ES, UK, USA
C. Peer-reviewed funding: During the period between 1st
Jan 1993 and July 2013, over £17
million funding was received by DDU with selected funding listed below:
1. World Health Organization, Nucleic acid based dipstick assay
for the detection of C.
trachomatis infection from urine. Awarded Sep 1995, $ 315,250
2. National Institutes of Health, USA, Nucleic acid dipstick for
Chlamydia trachomatis detection.
Awarded Jan 1996, £ 1,356,985
3. Sentinel Biosciences, Inc., Discovery of new HIV related
viruses in 3 West African countries.
Awarded Apr 1996, £ 1,764,138
4. Wellcome Trust Programme Grant: Development of a rapid DNA
dipstick for detection of
Chlamydia trachomatis. Awarded Mar 1997, £ 2,224,916
5. Wellcome Trust Translation Award: Evaluation of Chlamydia
trachomatis infection in clinical
settings. Awarded Feb 2005, £ 1,256,963
6. Wellcome Trust Strategic Award: Meeting the diagnostic needs
of resource-poor settings:
development of a simple amplification test, Awarded Jun 2007, £
2,885,434
7. National Institutes of Health, USA SAMBA HIV Point-of-Care
Nucleic Acid System for
Resource Limited Settings. Awarded Nov 2009, $4,654,673
8. UK Technology Strategy Board, Point-of-care nucleic acid-based
tests for detection of
Chlamydia trachomatis (CT) and Neisseria gonorrhea (NG),
Awarded Oct 2010, £841,327
9. Children's Investment Fund Foundation, Point
of care nucleic acid test for detection of HIV infections in infants.
Awarded Jul 2011, $4,917,586
Details of the impact
The two technology platforms (SAS for protein targets and SAMBA for
nucleic acid targets) have
been successfully commercialised by DDU's spinout company, Diagnostics for
the Real World
(DRW) established in 2002, with the Wellcome Trust and the University of
Cambridge as corporate
shareholders (Ref 1 in section 5). To meet the diagnostic needs of the
developing world, the
company limits its profit to 10%. In the past 10 years, the spinout
company has raised >$30 million
using a diversified funding strategy (Ref 2 in section 5), of which
>$20million is since 2008
(including $8million from NIH, $12million from the Children's Investment
Fund Foundation and
£0.5million from the UK Technology Strategy Board).
Both the female and the male Chlamydia SAS-based rapid tests have
undergone clinical trial in 3
UK clinics and in the Philippines (Refs 3 & 4 in section 5).). Since
their launch in 2008 at the
national OB-GYN conference in the Philippines, >150,000 tests have been
provided to diverse
geographic regions through its distribution partner (Oxoid Thermo-Fisher)
and direct sales: France,
Italy, the Czech Republic, Slovakia, Senegal, Madagascar, the Republic of
Niger, the Ivory Coast,
Morocco, Algeria, Tunisia, Kenya, Malaysia, the Seychelles, Vanuatu,
Samoa, the Falkland
Islands, Ghana, and St. Vincent and the Grenadines. They were used as a
diagnostic tool for
Chlamydia infection in symptomatic individuals as well as a screening tool
in pregnant women (e.g.
Samoa where Chlamydia is endemic), military personnel in the Falklands and
in asymptomatic
populations in general. The Chlamydia rapid test was funded by the UK
Technology Strategy
Board in 2012 as a tool to generate a cost effective model for `test and
treat' at the point of care vs.
centralised testing in 3 UK sexual health clinics. The FirstBurstf0e4
first void urine collection device
received the 2003 Medical Futures Best Diagnostic Innovation Award and
continues to be used in
the DRW Chlamydia test with on-going impact in studies such the UK
national survey for sexually
transmitted diseases in the general population.
DRW has also launched the first CE marked HBsAg rapid test kit on the
market due to its improved
sensitivity and has begun its launch activities in north African countries
and in France after a
successful evaluation. The test generated a large number of internet-based
articles during the
World Hepatitis Day in 2010, including comments from Prof Baruch Blumberg,
the Nobel Prize
laureate for the discovery of the Hepatitis B virus and invention of the
HBV vaccine: "Approval of
the new Hepatitis B Rapid Test is positive news for the estimated 400
million HBV carriers
worldwide. Being able to identify carriers, initiate immediate treatment
of appropriate candidates,
and vaccinate family members and close contacts, has the potential to
greatly accelerate the
programme to control HBV infection and spread. The Hepatitis B Rapid Test
developed by
Diagnostics for the Real World can make a significant contribution to the
solution" (Ref 5 in section
5).
The first assay based on the SAMBA platform is the HIV-1
Semi-Quantitative Test for the
monitoring of patients on antiretroviral therapy. Having been successfully
trialled by Medecins sans
Frontieres (MSF), the first batch of 4,000 tests and 10 SAMBA instruments
were shipped to 3 MSF
clinics in Malawi and Uganda in Q2, 2013. This is the first time a nucleic
acid amplification test is
implemented in a point-of-care setting. MSF has already committed to
screening >30,000 patients
for treatment efficacy using SAMBA. Product registration for the SAMBA
HIV-1 Semi-Q Test is
currently being sought in 6 other high-burden African countries (Cameroon,
Kenya, Nigeria, South
Africa, Zambia, Zimbabwe). Given the recent WHO recommendation to monitor
all HIV infected
patients at least once a year, the SAMBA HIV test will be an effective
tool for the monitoring of HIV
infected individuals in sub Saharan Africa (estimated to be 15 million in
2015).
Currently, HIV-exposed babies in sub-Saharan Africa can only be tested by
nucleic acid
amplification methods at centralised laboratories. This requires the
transport of dried blood spots
from peripheral clinics and leads to unacceptable delays to treatment due
to long turn-around times
and loss to follow up ranging from 30 to 70% depending on the country. A
quantitative SAMBA HIV
test has been developed for the early infant diagnosis at the point of
care, and access to this
simple, rapid and effective molecular test at peripheral clinics where
mothers can receive the
results in one visit will fill the current diagnostic gap. Ethical
approvals have been obtained in
Malawi, Uganda, South Africa and Kenya for clinical trials in Q3-Q4 2013.
A Memorandum of
Understanding was signed with the Ministry of Health, Zimbabwe for
implementation of the SAMBA
EID test.
Sources to corroborate the impact
-
Wellcome Trust Technology Transfer Showcase
http://www.wellcome.ac.uk/Funding/Technology-transfer/Technology-transfer-showcase/WTX052911.htm
-
Global Health Innovation Insight Series, Diagnostics for the
Real World - Raising funds for a
niche solution (2012)
http://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=6&ved=0CFIQFjAF&url=http%3A%2F%2Fwww.gsb.stanford.edu%2Fsites%2Fdefault%2Ffiles%2Fdocuments%2FDRWII-RaisingFunds.pdf&ei=T_95UrHQLfGM7Ab6y4GABA&usg=AFQjCNGSjqIxsnFJ3QZCMkZ-2fBPYuvBRA&sig2=oDcHxIjV7IAoSRRxHrLBRg&bvm=bv.55980276,d.ZGU
- L. Mahilum-Tapay, V. Laitila, J.J. Wawrzynia et al. New point of care
Chlamydia Rapid Test -
bridging the gap between diagnosis and treatment: performance evaluation
study. British Medical
Journal 2007. 335: 1190-1194.4.
- E-C. Nadala, B. T Goh, J-P. Magbanua, et al. Performance evaluation of
a new rapid urine test
for chlamydia in men: prospective cohort study British Medical
Journal 2009. 339:b2655;
doi:10.1136/bmj.b2655.
-
Wellcome Trust Press Release, 2010 "EU gives green
light for while-you-wait Hepatitis B test"
http://www.wellcome.ac.uk/News/Media-office/Press-releases/2010/WTX059435.htm