PREDICT: A prognostication and treatment benefit tool for early breast cancer - Caldas
Submitting Institution
University of CambridgeUnit of Assessment
Clinical MedicineSummary Impact Type
HealthResearch Subject Area(s)
Medical and Health Sciences: Oncology and Carcinogenesis, Public Health and Health Services
Summary of the impact
PREDICT is a prognostication and treatment benefit decision aid aimed at
aiding the breast cancer
multi-disciplinary team in the management of women with early breast
cancer. The user-friendly,
web-based tool was developed in collaboration with the Cambridge Breast
Unit multi-disciplinary
team, the Eastern Cancer Registration and Information Centre. Implemented
online, PREDICT is
hosted on a NHS web-server. Since 2012 PREDICT has been used widely by
clinicians
throughout the UK and world-wide.
Underpinning research
The PREDICT model was developed in 2010 by a team jointly led by
Professor Carlos Caldas
(University Professor 2006-present, in the department of Oncology) and
Professor Paul Pharoah
(UOA 2: CR-UK Senior Clinical Research Fellow 1999-2009, University Reader
2009-13,
University Professor 2013-present, in the department of Public Health and
Primary Care).
Patients treated in the Cambridge Breast Unit are stratified for adjuvant
chemotherapy according to
a guideline developed in 2004. This takes into account the serious adverse
events that occur with
chemotherapy and that as a consequence, "many physicians consider a
cut-off of an additional 3%
or more added benefit sufficient to justify recommending treatment". Thus,
for an absolute survival
benefit of < 3%, chemotherapy is not recommended, for an absolute
survival benefit of 3-5% the
benefits and harms are considered equivalent and discussed with the
patient, for an absolute
benefit of >5% chemotherapy is recommended.
Until 2010 the absolute benefits of chemotherapy were estimated using
Adjuvant! Online, an online
prognostic model developed over a decade ago by an American oncologist
(Peter Ravdin). This is
based on US data and has not been validated with UK data. Furthermore,
Adjuvant! Online does
not include several important prognostic variables including mode of
detection and molecular
biomarkers such as tumour HER2 status. Thus there was a clinical need for
an equivalent model,
based on and validated using UK data that was flexible and able to
incorporate additional
prognostic variables.
Research carried out in Cambridge allowed the development of the PREDICT
model, which was
based on survival-time data on 5,700 women with early breast cancer
treated between 1999 and
2003. These data were obtained through the Eastern Cancer Registration and
Information Centre
and used to determine the influence of key prognostic variables on
survival [1]. The model was
then validated using an independent data set from the West Midlands Cancer
Intelligence Unit [1].
The PREDICT web interface was developed in 2010 and is hosted by the
Eastern Cancer
Registration and Information Centre.
In order to compare directly the performance of Adjuvant! Online and
PREDICT, a second
validation of PREDICT was carried out using the same data set. While both
models performed
well, the breast cancer specific survival calibration for PREDICT was
significantly better than that
of Adjuvant! Online with similar discrimination [2].
In parallel with the PREDICT model development work, Caldas and Pharoah
have led a research
programme investigating the molecular pathology of breast cancer in
collaboration with other
groups from the international Breast Cancer Association Consortium (BCAC).
This research has
enabled further development of PREDICT in response to feedback from
clinicians and requests for
additional features in the model. In particular, there were many requests
to incorporate tumour
HER2 and KI67 status into the model. Results from one of the BCAC projects
were used to enable
the incorporation of HER2 into the model [3], with the new PREDICT model
being further validated
using the British Columbia data set used to validate the original model
[4]. More recently, the
results from another of our molecular pathology studies has informed the
incorporation of tumour
KI67 status into the model [5].
References to the research
1. Wishart GC, Azzato EM, Greenberg DC, Rashbass J, Kearins O, Lawrence
G, Caldas C,
Pharoah PD. PREDICT: a new UK prognostic model that predicts survival
following surgery
for invasive breast cancer. Breast Cancer Res. 2010;12(1):R1.
2. Wishart GC, Bajdik CD, Azzato EM, Dicks E, Greenberg DC, Rashbass J,
Caldas C,
Pharoah PD. A population-based validation of the prognostic model PREDICT
for early
breast cancer. Eur. J. Surg. Oncol. 2011;37(5):411-7.
3. Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling
J, Cheang MC,
Gelmon K, Nielsen TO, Blomqvist C, Heikkila P, Heikkinen T, Nevanlinna H,
Akslen LA,
Begin LR, Foulkes WD, Couch FJ, Wang X, Cafourek V, Olson JE, Baglietto L,
Giles GG,
Severi G, McLean CA, Southey MC, Rakha E, Green AR, Ellis IO, Sherman ME,
Lissowska
J, Anderson WF, Cox A, Cross SS, Reed MW, Provenzano E, Dawson SJ, Dunning
AM,
Humphreys M, Easton DF, Garcia-Closas M, Caldas C, Pharoah PD, Huntsman D.
Subtyping of breast cancer by immunohistochemistry to investigate a
relationship between
subtype and short and long term survival: a collaborative analysis of data
for 10,159 cases
from 12 studies. PLoS Med 2010;7(5):e1000279.
4. Wishart GC, Bajdik CD, Dicks E, Provenzano E, Schmidt MK, Sherman M,
Greenberg DC,
Green AR, Gelmon KA, Kosma VM, Olson JE, Beckmann MW, Winqvist R, Cross
SS,
Severi G, Huntsman D, Pylkas K, Ellis I, Nielsen TO, Giles G, Blomqvist C,
Fasching PA,
Couch FJ, Rakha E, Foulkes WD, Blows FM, Begin LR, Van't Veer LJ, Southey
M,
Nevanlinna H, Mannermaa A, Cox A, Cheang M, Baglietto L, Caldas C,
Garcia-Closas M,
Pharoah PD. PREDICT Plus: development and validation of a prognostic model
for early
breast cancer that includes HER2. Br. J. Cancer 2012;107(5):800-7.
5. Ali HR, Dawson SJ, Blows FM, Provenzano E, Leung S, Nielsen T, Pharoah
PD, Caldas C.
A Ki67/BCL2 index based on immunohistochemistry is highly prognostic in
ER-positive
breast cancer. J. Pathol. 2012;226(1):97-107.
Details of the impact
PREDICT is implemented online as a national resource with the web
interface being hosted on a
NHS web-server at www.predict.nhs.uk.
The monthly hits (> 4,000) on the website indicate clearly
the impact of the tool.
One of the key decisions in the management of women with early breast
cancer is whether or not
to offer adjuvant chemotherapy in conjunction with primary surgery and
radiotherapy. The key
output of PREDICT is the expected absolute reduction in mortality at five
and ten years associated
with adjuvant chemotherapy.
Cambridge Breast Unit (CBU)
Until 2010 the CBU estimated absolute benefits of chemotherapy were
estimated using Adjuvant!
Online. During 2010 and 2011 both PREDICT and Adjuvant! Online were used
in parallel.
Clinical audit
Demographically, the use of PREDICT could benefit 18% of women worldwide.
The Cambridge
Breast Unit carried out an audit of the first 200 patients discussed when
both Adjuvant! Online and
PREDICT were used by the multi-disciplinary team [1]. The chemotherapy
recommendations that
would have been made based on the output from each model were then
compared. In 163
patients (82 per cent) the chemotherapy decision would have been the same
whichever model was
used. A different recommendation would have occurred for 37 patients (18
per cent), which would
benefit them, some women avoiding unnecessary chemotherapy, others having
effective treatment
which would not otherwise have been given.
Change in practice
Since 2012 PREDICT has been the only model used routinely in Cambridge
for all patients being
discussed at the weekly multi-disciplinary team meeting. The absolute
benefit of adjuvant
chemotherapy estimated by PREDICT is used to guide the use of adjuvant
chemotherapy
according to the guideline described in section 2.
Other clinical departments in the UK
We have had multiple requests from clinicians for the incorporation of
additional features indicating
that the model is being widely used. PREDICT is used by the
multi-disciplinary clinical teams in
Belfast, Brighton, Derby, Dundee, Oxford and Sheffield, but the web usage
statistics from 2011
suggest that PREDICT is also being used widely across the country.
The impact of PREDICT on clinical practice is clearly demonstrated from
the extensive use of the
web interface (see below).
Public, Patient Partnership
PREDICT has been widely reported in regional and national media including
ITV, The Times and
The Daily Mail (ref 2). We have clear evidence that women with early
breast cancer are accessing
the interface in order to determine their own risk and to help them in
their discussion with their
oncologists about treatment options — this is very much in keeping with
current thinking to
empower patients through knowledge to play an important role in their own
care.
Web usage data
PREDICT was designed to have a user-friendly interface to help clinicians
in making clinical
management decisions. Informal feedback from clinicians from both
Cambridge and elsewhere
has indicated that the interface is easy to use.
The number of visits to the web site each month has increased steadily
since its launch in January
2011, with 3,266 visits in April 2013.
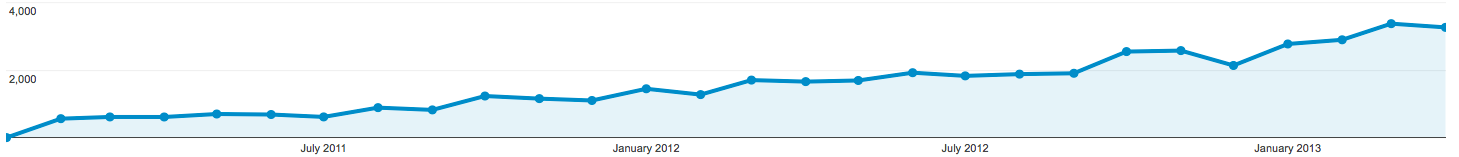 Monthly web usage statistics for the PREDICT website, Jan 2011-Jan
2013
Monthly web usage statistics for the PREDICT website, Jan 2011-Jan
2013
There have been 43,870 visits to the web site with 70 per cent of the
visits (30,563) being
accessed from UK and ten per cent (4,468) from USA. The web site is
visited from all over the UK,
with London accounting for 17 per cent and Cambridge accounting for just 1
per cent of all traffic
on the web site (ref 3).
Sources to corroborate the impact
- Loh S-W, Rodriguez-Miguelez M, Pharoah P, Wishart G. A
comparison of chemotherapy
recommendations using the Predict and Adjuvant models. Eur. J. Surg.
Oncol.
2011;37(5):S21-S22.
- Press coverage: see http://www.predict.nhs.uk/press.shtml
for details.
- Web usage statistics from Google Analytics at https://www.google.com/analytics