The Prostate Core Mitomic Test: a commercial diagnostic to improve the efficiency of prostate cancer diagnosis
Submitting Institution
Newcastle UniversityUnit of Assessment
Biological SciencesSummary Impact Type
TechnologicalResearch Subject Area(s)
Biological Sciences: Genetics
Medical and Health Sciences: Oncology and Carcinogenesis
Summary of the impact
A novel test for prostate cancer was developed from research in
mitochondrial genetics conducted at Newcastle University. The Prostate
Core Mitomic Test was the first of its kind and is now commercially
available in North America. It provides molecular evidence to confirm
conventional pathology results showing that men identified as being at
risk of prostate cancer are, at the time of examination, free of disease.
This is an important patient benefit, as conventional pathology has a 30%
chance of missing prostate cancer. The Mitomic test obviates the
short-term need for a follow-up biopsy, which is an invasive and very
uncomfortable procedure. It is also capable of identifying some men at
high risk of having prostate cancer that conventional pathology would
miss. The test was introduced to the American market in June 2011 and has
generated a multi-million dollar investment and turnover.
Underpinning research
Researchers
Professor Mark Birch-Machin is a dermatologist with a background in
mitochondrial genetics research at Newcastle University, and is a
co-founder and a director of Genesis Genomics Inc. (since renamed
Mitomics Inc.), together with Drs Ryan Parr and Robert Thayer, who
are based in Canada. Birch-Machin was Principal Investigator on a Cancer
Research UK study, and Dr Andrew Harbottle was the Research Associate
(Harbottle joined Mitomics in 2005).
Background: prostate cancer
Prostate cancer is the second most common cancer in males worldwide, and
the fifth most common cancer overall. Incidence rates vary depending on
country and ethnicity, but the American Cancer Society reports a rate of
192,280 new cases per year and it is estimated that more than 2,600,000
men are living with the disease in the USA. The symptoms of prostate
cancer are often similar to less serious conditions such as benign
prostate enlargement; hence reliable diagnosis of malignant disease is
important. The current best practice for reliable diagnosis is needle
biopsy at 12 different locations in the prostate for microscopic
examination by a pathologist (see Figure 1). However, this method relies
on at least one of the biopsies taken hitting the tumour and prostate
cancer often presents as multiple small tumours, rather than a single
large mass. Consequently a negative result may be false. If blood tests
and other indications still suggest a high risk of disease being present
then a second biopsy procedure would be undertaken after a short time.
Background: mitochondria
Mitochondria are cellular structures that provide 90% of the body's
energy requirements. They have their own DNA (the mitochondrial genome),
which is highly susceptible to damage compared to nuclear DNA. This is
because mitochondrial DNA lacks protective proteins and it is continually
exposed to reactive oxygen species generated by cell respiration. It also
has limited capacity for repair, unlike nuclear DNA. There are many
different mitochondrial genomes in a cell, typically more than a thousand.
This redundancy means that mitochondrial genomes can tolerate high levels
(up to 90%) of damaged DNA. This can lead to the accumulation of genetic
damage without cell function being compromised. One source of DNA damage
is a so-called `field effect' that surrounds malignant tumours; this
concept was developed by D.P. Slaughter in 1953 (Cancer, PMID:
13094644). Tumour field effects are molecular changes in apparently benign
cells located at a distance from a tumour.
Research
In 2003, Newcastle published the first detailed study of the distribution
of multiple forms of mitochondrial DNA damage in non-melanoma skin cancer
(R1). As well as identifying point mutations in the mitochondrial genome,
this work provided quantitative data of the incidence of a common deletion
of part of the mitochondrial DNA. Later work (R2) refined the methodology,
and identified a practical application related to skin cancer and exposure
to sunlight.
This research suggested that such mitochondrial DNA deletions may also be
found in other cancers elsewhere in the body. By sequencing the whole
mitochondrial genome from prostate cells taken from patients who had
advanced prostate cancer, and comparing the results with those from
patients who were free of disease, the researchers in Newcastle and Canada
showed that deletions in the mitochondrial genome were not restricted to
overtly cancerous tissue (R3, R4). Cells located at a distance, in healthy
looking tissue, as well as those adjacent to tumours, carried the
mitochondrial DNA changes that were also found in the cancer; this field
effect is the basis of the Prostate Core Mitomics Test.
References to the research
(Newcastle researchers in bold. Citation count from Scopus, July 2013)
R1. Durham SE, Krishnan KJ, Betts J and Birch-Machin MA (2003)
Mitochondrial DNA Damage in Non-Melanoma Skin Cancer. British Journal
of Cancer 88:90-5. doi:10.1038/sj.bjc.6600773 Cited by 54.
R2. Harbottle A and Birch-Machin MA (2006) Real-time PCR
analysis of a 3895 bp mitochondrial DNA deletion in nonmelanoma skin
cancer and its use as a quantitative marker for sunlight exposure in human
skin. British Journal of Cancer 94:1887-93.
doi:10.1038/sj.bjc.6603178 Cited by 17.
R3. Parr RL, Dakubo GD, Crandall KA, Maki J, Reguly B, Aguirre A, Wittock
R, Robinson K, Alexander JS, Birch-Machin MA, Abdel-Malak M,
Froberg MK, Diamandis EP and Thayer RE (2006) Somatic Mitochondrial DNA
Mutations in Prostate Cancer and Normal Appearing Adjacent Glands in
Comparison to Age-Matched Prostate Samples without Malignant Histology. The
Journal of Molecular Diagnostics 8(3):312-9. http://dx.doi.org/10.2353/jmoldx.2006.050112
Cited by 36.
R4. Parr RL, Dakubo GD, Thayer RE, McKenney K and Birch-Machin MA
(2006) Mitochondrial DNA as a potential tool for early cancer detection. Human
Genomics, 2(4):252-7. doi:10.1186/1479-7364-2-4-252 Cited by 19.
Funding
Cancer Research Campaign: 2001-2003 Mutations and deletions of the
mitochondrial genome in non-melanoma skin cancer. Dr MA
Birch-Machin, Dermatology, University of Newcastle, £82,400 (including
£7,800 supplementation May 2002).
Details of the impact
The identification of a tumour field effect on mitochondrial DNA in
prostate tissue resulted in the development of a commercially available
test (launched in the USA in March 2011) that has benefitted men at risk
of prostate cancer. This test works by: (i) reducing the need to have a
second prostate biopsy by confirming their disease-free status, and (ii)
identifying malignant disease in biopsy samples that appear healthy
visually (www.mitomicsinc.com/prostate-core-mitomic-test/). A further
impact is the expansion of a private company co-founded by Birch-Machin,
in which Newcastle University is a shareholder.
Pathway to impact: Newcastle influence on product development
The chief executive officer of Mitomics says of Birch-Machin's
contribution that:
In his role as chair of the company's Science Advisory Board and the
science management committee, Birch-Machin was able to advise the
background development of the science that eventually led to the
prostate cancer test, drawing on his work on skin cancers and the
correlation with mitochondrial DNA deletions. (Ev a)
The design of both the mitochondrial DNA sequencing and analysis
strategies was led by researchers at Newcastle University and based on
Birch-Machin's approaches in skin cancer research. Birch-Machin also
provided specialist advice on the review of mitochondrial DNA data that
led to the final version of the diagnostic test (Ev a). The test was
validated by the US National Institute of Standards and Technology under
the Early Detection Research Network of the National Cancer Institute,
following an external study conducted by them on 108 prostate biopsy
samples in 2008 (Ev b & Ev c).
Patents
The technology underlying the final test has been protected by patents,
on which Birch-Machin and Harbottle are named co-inventors. Filed in 2006
and granted in 2010, European patent number EP1877559B1 `Mitochondrial
mutations and rearrangements as a diagnostic tool for the detection of
sun exposure, prostate cancer and other cancers' protects the
technology behind the methods and kits used to reveal mitochondrial DNA
deletions and permit the early detection, diagnosis and progression of
prostate cancer, sun exposure and non-melanoma skin cancer (Ev d). A
patent (US8008008B2) filed in 2007 covering the specific mitochondrial
biomarker and its application for the detection of prostate cancer was
granted in the USA in 2011 (Ev e).
The Prostate Core Mitomic Test and patient benefit
As noted earlier, confirming a diagnosis of prostate cancer relies on a
pathologist identifying the disease in one or more of 12 needle biopsies
as shown in Figure 1. It is estimated that in 2011 there were 1,498,000
prostate biopsies performed in North America. On average, 70% of biopsies
(around 1 million) show a negative result, and around 30% of these will be
false negatives (Ev f).
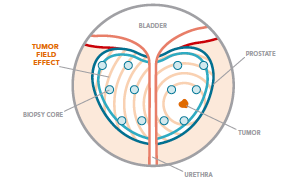 Figure 1. Schematic of the prostate gland, needle biopsy strategy and the
tumour field effect.
Figure 1. Schematic of the prostate gland, needle biopsy strategy and the
tumour field effect.
Using the same samples taken for pathology examination, The Prostate
Core Mitomic Test makes use of the tumour field effect (shown in
Figure 1) by identifying a particular mitochondrial DNA deletion in
visually benign cells (Ev c).
It is sometimes the case that men whose cells appear disease-free on
pathological examination still have raised levels of prostate-specific
antigen (a protein in the blood associated with prostate cancer). However,
in a clinical study involving 101 patients, the Prostate Core Mitomic
Test identified those men who were truly free of disease with a
negative predictive value of 91% by confirming the lack of a tumour field
effect, and identified patients at high risk for undiagnosed prostate
cancer at a sensitivity of 84% (Ev g). As the test is successful in
identifying men at low risk who would otherwise require a follow-up biopsy
procedure in the short term, there is an important patient benefit. The
test's use of existing samples reduces stress and the risk of infection as
no further biopsies are required to confirm the diagnosis. The test also
identifies high risk patients undiagnosed with conventional biopsy; it
identified 17 of 20 patients who were later diagnosed with prostate
cancer.
Financial investment and commercial impact following product launch in
2011
The chief executive officer of Mitomics has confirmed in July 2013 that:
As a private company, we do not disclose financial information
publicly. However, Mitomics has invested significant sums into the
development of Prostate Core Mitomic Test... The launch of the product
required the hiring of a sales and marketing team, as well as the
establishment of a commercial laboratory for test processing. The
combined peak staffing of the USA team is fifteen people. (Ev a)
The product development phase was funded by multi-million dollar
investment from existing shareholders and private equity management
companies. Following the launch of the test onto the American market,
sales of the Prostate Core Mitomics Test have grown in line with
forecasts and have reached several million dollars (Ev h).
Mitomics has entered into licence agreements with six companies
with established networks of urologists in private and public healthcare
(Ev i). These include LabCorp (one of the world's largest clinical
laboratory providers, which has an annual revenue of $5.7 billion) and CML
HealthCare (recently the subject of a takeover, in which the company was
valued at $917 million).
Mitomics has twice (in 2007/8 and 2010/11) been selected as one of
Canada's top 10 private companies in the life sciences sector. Winners of
this competition are chosen by an independent expert panel of leading
Canadian and US venture capitalists. Competition winners participate in a
series of investment forums across the USA, providing access to potential
strategic partners (Ev j).
Sources to corroborate the impact
Ev a. Letter from the chief executive officer of Mitomics Inc. Contact
details are available on request should corroboration of evidence be
required.
Ev b. Maki et al. (2008) Mitochondrial Genome Deletion Aids in the
Identification of False- and True-Negative Prostate Needle Core Biopsy
Specimens. American Journal of Clinical Pathology 129:57-66. DOI:
10.1309/UJJTH4HFEPWAQ78Q.
Ev c. Study data is shown at http://www.mitomicsinc.com/prostate-core-mitomic-test/
and can be found in the downloadable `white paper'.
Ev d. The EU patent can be viewed at: http://www.google.com/patents/EP1877559B1?cl=en
Ev e. The USA patent can be viewed at https://www.google.com/patents/US8008008
Ev f. American data on prostate cancer screening is available at
http://www.cancer.org/acs/groups/content/@nho/documents/document/500809webpdf.pdf
Ev g. Robinson et al (2010) Accurate prediction of repeat prostate cancer
biopsy outcomes by a mitochondrial DNA deletion assay. Prostate Cancer and
Prostatic Diseases 13:126-31. DOI: 10.1038/pcan.2009.64
Ev h. Mitomics Inc is a private company and as such does not disclose
detailed financial information. However, the Chief Executive Officer may
be contacted should confirmation of the multi-million dollar nature of
investment and sales be required. Shareholder information is routinely
available to Newcastle University but is commercially sensitive. Newcastle
University is a minority shareholder and as such is unable to influence
the commercial decision not to make financial details available in this
impact case, but the University as a shareholder does have access to
company financial statements that can be accessed on request, but not
copied or otherwise shared.
Ev i. Press releases confirming the licensing agreements reached with six
large distribution companies are available at http://www.mitomicsinc.com/media-center/press-releases.php
Ev j. The competition website, listing Mitomics among the winners, is
available at http://www.topcanadiancompanies.com/winning_companies.html