Improving success rates in the surgical treatment of cataract
Submitting Institution
University of East AngliaUnit of Assessment
Biological SciencesSummary Impact Type
TechnologicalResearch Subject Area(s)
Medical and Health Sciences: Ophthalmology and Optometry
Summary of the impact
Cataract causes blindness in millions of people worldwide. It is treated
surgically by replacing
the clouded lens with an artificial lens and more than 30 million such
operations per year are
predicted by 2020. Unfortunately, many of these patients are subsequently
blighted by posterior
capsule opacification (PCO) a wound-healing response by lens epithelial
cells to surgical
trauma. Using human donor eyes, Wormstone and Duncan developed a technique
that
simulated cataract operations and provided an ideal system to understand
PCO biology. This
technology was a key platform in developing a novel commercial intraocular
lens (IOL), which
shows massive reductions in PCO rates.
Underpinning research
Cataract renders tens of millions blind. In addition to reducing the
quality of an individual's life,
significant healthcare provision is required. Currently, the only means to
treat cataract is by
surgical intervention. Often, and especially in children, cataract surgery
is blighted by a fibrotic
condition known as Posterior Capsule Opacification (PCO), due to a
wound-healing response to
surgical trauma by lens epithelial cells, which reduces visual quality.
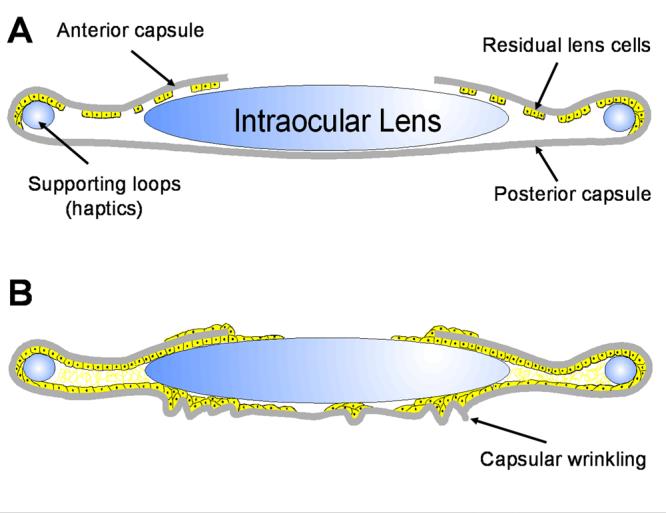
The image below shows a
representation of (A) the post surgical capsular bag and (B) the extensive
growth and
modification that causes PCO following cataract surgery.
Cataract surgery requires the creation of a
circular opening (a process known as
capsulorhexis) in the front part of the lens
(anterior capsule). This opening allows
access to the central fibre cells within the
lens, which are typically those affected by
cataract. The remaining lens tissue,
comprising a ring of anterior lens capsule
and the entire posterior capsule, is known
as a Capsular Bag (CB), which maintains
separation of the aqueous and vitreous
humours. This bag can house an artificial
intraocular lens (IOL), which is commonly
implanted during surgery and restores the
refractive power that is lost by fibre cell
removal. Immediately following surgery, a marked improvement in visual
quality is observed
because light can pass through the lens capsular bag without meeting
light-scattering structures.
Unfortunately, in many patients the lens epithelial cells that line the
anterior capsule survive and
grow. Importantly, these cells grow on to the cell-free posterior capsule,
causing them to
encroach upon the visual axis. A thin cover of cells is insufficient to
affect the light path, but
subsequent changes to the matrix, cell organisation and phenotypic shift
(trans-differentiation)
give rise to light scatter. This markedly decreases visual quality and
normally requires more
surgery. This disruption of the posterior capsule is known as PCO;
patients of all ages are
affected, but it is particularly severe in children.
To study PCO progression, Duncan, Wormstone and colleagues working at UEA
developed an
innovative in vitro system that employed a simulated cataract
operation on human donor eyes to
produce a CB [1]. This can be cultured in controlled environmental
conditions and monitored on
a day-to-day basis. This allowed several clinical features of PCO to be
replicated, including age-related
wound-healing rates [2], matrix contraction, matrix deposition and
phenotypic shift from
epithelial cells to myofibroblasts [4]. Moreover, the effects of exogenous
growth factors and
autocrine factors on these characteristics were evaluated [4, 5, 6]. Also,
IOLs were implanted
into the capsular bag and their influence on PCO was assessed [1, 3].
References to the research
1) Liu CSC, Wormstone IM, Duncan G, Marcantonio JM, Webb SF,
Davies PD (1996). A
study of human lens cell growth in vitro: a model for posterior
capsule opacification. Invest.
Ophthalmol. Vis. Sci. 37: 906-914. (104 Citations)
http://www.iovs.org/content/37/5/906.abstract
2) Wormstone IM, Liu CSC, Rakic J-M, Marcantinio JM,
Vrensen GFJM, Duncan G (1997).
Human lens epithelial cell proliferation in protein-free medium. Invest.
Ophthalmol. Vis.
Sci. 38: 396-404. (91 Citations)
http://www.iovs.org/content/38/2/396.full.pdf
3) Duncan G, Wormstone IM, Liu CSC, Marcantonio JM,
Davies PD (1997). Thapsigargin
coated intraocular lenses inhibit human lens cell growth. Nature
Medicine 3:1026-1028.
(85 Citations)
doi: 10.1038/nm0997-1026
4) Wormstone IM, Tamiya S, Marcantonio JM, Reddan JR. (2000)
Hepatocyte growth
factor function and c-met expression in human lens epithelial
cells. Invest Ophthalmol Vis
Sci. 41:4216-4222. (47 Citations)
http://www.iovs.org/content/41/13/4216.abstract
5) Wormstone IM, Del Rio-Tsonis K, McMahon G, Tamiya S,
Davies PD, Marcantonio, JM,
Duncan G. (2001) FGF: an autocrine regulator of human lens cell growth
independent of
added stimuli. Invest Ophthalmol Vis Sci. 42:1305-1311.
(35 Citations)
http://www.iovs.org/content/42/6/1305.abstract
6) Wormstone IM, Tamiya ST, Anderson I, Duncan G. (2002)
TGF 03b22 induced matrix
modification and cell transdifferentiation in the human lens capsular bag.
Invest
Ophthalmol Vis Sci. 43: 2301-2308. (120 Citations)
http://www.iovs.org/content/43/7/2301.long
Key Grants
Since 1994, Prof. Duncan and Dr Wormstone have received continuous
funding (total > £1.9
million) from The Humane Research Trust to support their work on human
tissue. Also, projects
that have employed the capsular bag model and which aided its development
have been
supported (total ~£750K) by BBSRC, Cambridge Antibody Technology, Fight
for Sight, the Lord
Dowding Fund, the Dunhill Medical Trust and the James Tudor Foundation.
Details of the impact
The innovative human CB model developed by Wormstone and Duncan at UEA
has played a
crucial role in the development of a new IOL, known as the
bag-in-the-lens (BIL). BIL is
commercially available from Morcher Implants (Product name: Type
89) and to date Morcher
have sold 12,651 units worldwide (corroborating source A). Surgical
implantation of BIL
markedly reduces the incidence of PCO.
Because the CB model is generated through a simulated operation in the
laboratory, it is
essentially the same as that generated in a cataract patient. This makes
it an ideal platform for
testing novel clinical concepts in a regulated environment, which is
amenable to ongoing
observation and analysis. This led the Medical Director at the University
Hospital Antwerp to use
the Wormstone/Duncan CB model to evaluate the novel BIL. She knew of the
CB model and its
potential through published papers (section 3) and through discussions
with Wormstone and
Duncan at academic conferences. These discussions provided further
assistance in applying the
CB model to test the BIL, whose introduction into the eye requires a
technically demanding
surgical approach. This was of major value, as shown in the testimonial
below:
"I have personally employed the capsular bag model to great effect in
the development
of the BIL design and believe it has been an invaluable tool in
translating my original
concept into a device that has improved the lives of tens of thousands
of cataract
patients."
(corroborating source B)
Using the Wormstone/Duncan CB model, the skills needed for this surgical
procedure could be
developed and honed, as follows. In contrast to conventional cataract
surgery, which requires a
single capsulorhexis (capsular tear) in the anterior lens capsule, the BIL
technique involves the
use of a twin capsulorhexis lens design, and performance of anterior and
posterior
capsulorhexes of the same size. According to this concept, if both
capsules are well-stretched
around the optic of the lens, any remaining lens epithelial cells will be
captured within the
remaining space of the capsular bag, and their proliferation will be
limited to this space, so the
visual axis will remain clear. The CB model allowed it to be ascertained
that the BIL surgical
technique and implantation could be applied to human lenses, and was
therefore appropriate for
cataract patients. Moreover, the model allowed comparison with
conventional IOL designs and
thus demonstrated the ability of BIL to prevent PCO formation and show
that the BIL is a major
advance on existing devices (corroborating sources A-C).
The beneficiaries are those patients that have had surgical
implants with this new technique and
who will not experience deterioration in vision nor require further
surgery because of PCO. The
BIL is surgically implanted into ~5000 adult and child patients a year in
Europe alone, effectively
restoring their vision and the Medical Director at the University Hospital
Antwerp has personally
implanted >8000 since the lens received European CE mark approval in
2004. Significant
numbers of BILs have been implanted now and the outcomes are impressive. Of
particular
interest is the application to children, who have rapid onset of
blinding PCO; in nearly all
cases; the BIL prevents PCO, even in these extreme cases. The
limiting factor in the uptake
of this approach is the level of skill required by the surgeon to carry
out this procedure, a
challenge to many surgeons. Implanting BIL is not standard practice and
thus is not
conventionally taught. To address this issue, and to increase the pool of
surgeons using the BIL
procedure, wet lab and instructional courses are now run at the annual
European Society of
Cataract and Refractive Surgeons conference. In addition, an international
panel of BIL
Instructors has been established who pass on their knowledge and
demonstrate the technique
(corroborating source B). The weight of clinical data is strong and
numbers employing BIL are
growing.
Wormstone is also testing novel IOLs for Anew Optics using the CB
model. Data generated
using this model is aiding development of new IOL designs and their
selection for clinical trials.
In reference to this work, the CEO of Anew Optics has stated:
"The findings were revealing and have made a major impact on our
strategies and
approach in future clinical trials. In essence, I consider your work in
the capsular bag
pivotal for evaluating the performance attributes of new technologies."
(corroborating source D)
Sources to corroborate the impact
A. Bag-in-the-lens (BIL) documentation from Morcher Implants:
(i) email from Morcher implants giving sales numbers of the BIL
at 12,561 worldwide — held
on file at UEA.
(ii) videos showing the BIL implantation procedures:
http://www.morcher.com/videos/bag-in-the-lens/
(iii) details of the BIL and the surgical training courses available:
http://www.morcher.com/fileadmin/content/Broschueren_Kataloge/CATALOG-89A-TASSIGNON_2012-05-02.pdf
(iv) more details of instructional courses for BIL:
http://www.morcher.com/en/produkte/bag-in-the-lens.html
B. Corroborating letter, held on file at UEA, from the Medical Director,
Chair & Head of the
Department of Ophthalmology, University Hospital Antwerp, who developed
BIL and
regularly implants it into patients.
This letter describes how Wormstone and Duncan's capsular bag model
was used to
invented the bag-in-the-lens surgical technique. It also states that BIL
has been
implanted into 8000 patients since approval of the lens by the Belgian
Social Security in
2004. It is now estimated that ~5000 patients a year are implanted with
BIL IOLs in
Europe.
C. Key publications referring directly to the use of the CB model:
(i) De Keyzer K, Leysen I, Timmermans JP, Tassignon MJ (2008). Lens
epithelial cells in
an in vitro capsular bag model: lens-in-the-bag versus bag-in-the-lens
technique. J
Cataract Refract Surg 34:687-695. doi: 10.1016/j.jcrs.2007.11.055
This study evaluated the difference in lens epithelial cell (LEC)
growth between lens-in-the-bag
(traditional IOL) implantation and bag-in-the-lens (novel IOL)
implantation using
the in vitro human capsular bag model described by Liu et al 1996.
(ii) De Groot, Vrensen GFJM, Willekens B, Van Tenten Y, Tassignon MJ
(2003). In Vitro
Study on the Closure of Posterior Capsulorrhexis in the Human Eye. Invest.
Ophthalmol.
Vis. Sci. 44: 2076-2083. doi: 10.1167/iovs.02-0525
This work studied the closure of the posterior rhexis zone in an in
vitro capsular bag
model, described by Wormstone et al 1997.
D. Corroborating letter, held on file at UEA, from the CEO of Anew
Optics which states:
"We have employed the capsular bag model to assess modified IOL
designs under
different environmental conditions and their ability to prevent PCO like
changes. The
findings were revealing and have made a major impact on our strategies
and approach
in future clinical trials. In essence, I consider your (Wormstone's)
work in the capsular
bag pivotal for evaluating the performance attributes of new
technologies."