A Wearable Light Source for Ambulatory Treatment of Skin Cancer and Acne
Submitting Institutions
University of St Andrews,
University of EdinburghUnit of Assessment
PhysicsSummary Impact Type
TechnologicalResearch Subject Area(s)
Chemical Sciences: Macromolecular and Materials Chemistry
Engineering: Materials Engineering
Technology: Nanotechnology
Summary of the impact
Impact: Health and Economic Gains:
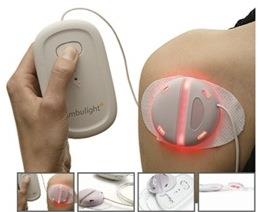
Research has led to a wearable light source that provides a new way of
treating many skin cancers and acne. The treatment is safe, convenient,
and easy to use bringing benefits to patients and healthcare providers. In
addition it brings economic benefits to Ambicare Health Ltd, the company
commercialising it.
Significance
For skin cancer treatment, the device gives effective treatment with much
reduced pain. The simplified treatment procedure allows more patients to
be treated in a clinic session. For acne, the device provides a convenient
at-home treatment without the application of drugs or chemicals.
Beneficiaries:
Skin cancer and acne sufferers, the clinics that treat them and Ambicare
Health Ltd.
Attribution:
The work was led by Professor Ifor Samuel (PHYESTA) working with
Professor James Ferguson (Ninewells Hospital, Dundee).
Reach:
The wearable light source has changed treatment in the UK and the
Netherlands. The skin cancer treatment is in regular use at more than 25
clinics, and the acne treatment at more than 250 clinics.
Underpinning research
Research in optoelectronics has been a major activity in PHYESTA in
recent times. An important aspect of Prof Samuel's work since 2000 has
been organic semiconductor optoelectronics, including organic
light-emitting diodes (OLEDs). OLEDs are compact visible light sources
with the potential to be flexible. They consist of thin layers of organic
semiconductors in between suitable contacts. Research in the Organic
Semiconductor Centre covers many aspects of these devices — materials,
photophysics, device physics, optical design and applications. Materials
research focuses on solution-processed materials such as conjugated
dendrimers and polymers. Photophysics concerns the formation and nature of
the excited state responsible for light emission. Device physics includes
charge injection and transport, and optical design concerns light
out-coupling and ways of manipulating it. This research has led to
understanding of the factors controlling OLED efficiency and routes to
improve it, together with the capability to make efficient devices to a
high standard [R1, R2].
It is known that light, in combination with a photosensitiser can be used
to treat many cancers, a process called photodynamic therapy (PDT). In the
case of skin cancer, a cream is applied to the lesion to be treated, and
the cream is metabolised to the photosensitiser. The photosensitiser is
then illuminated by a powerful light source (often a laser) leading to the
tumour being destroyed. This procedure gives very good cosmetic outcome,
though requires spending a day at the hospital and can be painful. The
need for specialised equipment means that relatively few centres (7 in
Scotland) are able to offer this treatment. A discussion with Prof
Ferguson, Head of Photobiology at Ninewells Hospital led to the idea of
using a wearable (and disposable) light source instead of the current
bulky and expensive hospital-based light sources, and a patent on this
invention was filed late in 2001 [R3], and an alternative implementation
filed in 2006 [R4].
In subsequent research, a major part of which was supported by proof of
concept funding from Scottish Enterprise, wearable light sources suitable
for medical use were made in St Andrews and evaluated at Ninewells
hospital, The concept of an ambulatory light source for medical and
cosmetic purposes was implemented using both organic and inorganic
light-emitting diodes [R4]. The former gives a more compact light source
and more uniform illumination; the latter is easier to manufacture in a
conventional electronics factory. After initial clinical evaluation [R5],
the OLED device was successfully used in a pilot trial that showed
equivalent effectiveness and much-reduced pain in the treatment of skin
cancer [R6].
The above research was a major part of research recognised through the
award of the Beilby Medal and Prize to Prof Samuel. This medal is awarded
by the Institute of Materials, the Royal Society of Chemistry and the
Society for the Chemical Industry for materials research of exceptional
practical significance. International recognition of its practical
significance came through the Organic Semiconductor Centre winning the
Academic R&D award at Printed Electronics USA, the world's largest
printed electronics meeting.
Personnel:
Key PHYESTA researchers involved were Professor Ifor Samuel
(2000-Present) with Dr Miguel Camacho Lopez (PDRA 2002-2003) and Andrew
McNeill (PDRA 2003-2007)
References to the research
The quality of the underpinning research is best indicated by R1, R2 and
R6. [Number of citations]
| [R1] |
J.P.J. Markham, S-C. Lo, S.W. Magennis, P.L. Burn and
I.D.W. Samuel , “High efficiency
green phosphorescence from spin-coated single-layer dendrimer
light-emitting diodes”, Applied Physics Letters 80,
p. 2645, (2002), DOI: 10.1063/1.1469218, URL: tinyurl.com/kyqmcng,[172]
|
| [R2] |
S.C. Lo, N.A.H. Male, J.P.J. Markham, S.W. Magennis, P.L. Burn,
O.V. Salata and I.D.W. Samuel, ‘A green phosphorescent dendrimer for
light-emitting diodes’,Advanced Materials, 14,
p. 975 (2002) DOI:
10.1002/1521-4095(20020705)14:13/14<975::AID-ADMA975>3.0.CO;2-D
URL: tinyurl.com/mfqg55v, [250]
|
| [R3] |
I.D.W. Samuel and J. Ferguson, ‘Therapeutic
light-emitting device’, UK patent application GB20010027581
filed 17/11/2001 and associated international applications |
| [R4] |
I.D.W. Samuel, J. Ferguson and A.P. McNeill, ‘Light-emitting
device for use in Therapeutic and/or Cosmetic Treatment’ UK patent
application GB20060008315 filed 27/06/2006 and associated
international applications |
| [R5] |
H. Moseley, J.W. Allen, S. Ibbotson, A.
Lesar, A. McNeill, M.A. Camacho-Lopez, I.D.W. Samuel, W. Sibbett and
J. Ferguson, ‘Ambulatory
photodynamic therapy: A new concept in delivering photodynamic
therapy’, British Journal of Dermatology, 154,
p. 747 (2006), DOI: 10.1111/j.1365-2133.2006.07145.x, URL:
tinyurl.com/k2ttz5h, [25]
|
| [R6] |
S.K. Attili, A. Lesar, A. McNeill, M. Camacho-Lopez, H. Moseley,
S. Ibbotson, I.D.W. Samuel and J. Ferguson, ‘An
open pilot study of ambulatory photodynamic therapy using a
wearable low-irradiance organic light-emitting diode light source
in the treatment of nonmelanoma skin cancer’, British
Journal of Dermatology, 161,
p.170 (2009), DOI: 10.1111/j.1365-2133.2009.09096.x, URL:
tinyurl.com/n8ctfv2, [23]
|
Details of the impact
The research on OLEDs including device fabrication capabilities provided
the opportunity to make compact wearable light sources for skin cancer
treatment. In order to realise this vision (as mentioned briefly above) we
applied for and received a "proof of concept" grant from Scottish
Enterprise to make a demonstrator device whose initial evaluation was
conducted at Ninewells Hospital. Further support from Scottish Enterprise,
led to the fabrication of further devices in St Andrews, which were used
in a pilot trial demonstrating the potential of OLEDs for the photodynamic
therapy of skin cancer.
In order to enable the above research to be widely used, the prototype
devices made in the research outlined above needed to be developed into a
form suitable for regulatory approval and manufacture. The regulatory
approval of a medical device is a major task requiring extensive design
and testing to appropriate standards and so is both expensive and time
consuming. In order to address this, a spin-out company, Ambicare Health
Ltd was formed, and £2M of venture capital raised a the start of 2008.
This funded the development of skin cancer and acne products following
the ISO13485 standards for medical devices and leading to CE marking of
both devices, thereby enabling them to be sold in all countries of the
European Union [S1]. In addition the skin cancer treatment has regulatory
approval in Australia, a major market for skin cancer treatment. A further
£2M of venture capital has been raised since to support the manufacture
and marketing of these products. The product for skin cancer treatment is
called "Ambulight" and the acne treatment is called "Lustre" [F1]. The
official unveiling of the Ambulight product led to a wide range of press
and media interest and featured in many major UK and international
newspapers and on popular TV shows. [S2]
The research has led to health and economic benefits. The economic impact
to date is primarily in the form of licence, assignment and royalty fees
paid by Ambicare Health and totalling [text removed for publication]. By
simplifying treatment, and increasing the number of patients treated per
clinic (see below), there are also economic benefits to the treatment
provider (e.g. NHS) but we have not been able to quantify these.
The healthcare benefits of Ambulight are described in a letter by the
Joint Head of Photobiology at Ninewells Hospital [F2], who was not
involved in the development of the device, but has been performing
research on its effectiveness and is in charge of delivering Photodynamic
Therapy at the hospital. In the letter she explains that conventional PDT
is an effective treatment, but the light sources "include expensive
lasers and relatively cumbersome static polychromatic, predominantly LED
sources". She explains "there are limitations with hospital-based
PDT and these include the fact that only a limited number of patients
can be treated in any one clinic session because of the availability of
specialised hospital-based light sources; the patient needs to wait for
a 3 hour period whilst the cream is in place and therefore this involves
at least a half-day visit to the hospital' the high intensity of the
light delivery using the hospital sources causes pain which in
approximately 16-20% is severe........ Pain has resulted in patients
discontinuing treatment prematurely and therefore not having effective
therapy .....It also limits the wider acceptance of
PDT....Hospital-based irradiation also requires that the patient must
lie still. Eye protection is needed for both the patient and staff.."
The Ambulight device overcomes these limitations, providing a convenient
and comfortable treatment with advantages for both the patient and the
treatment provider. She explains "The Ambulight device has
revolutionised many of the problems we have with conventional PDT. It is
a portable, wearable light source with battery pack. This means that it
is ideally suited for patients who are mobile and/or keen to have
treatment at home. It also considerably reduces the amount of time that
the patient must attend the hospital and reduces the amount of input
from staff such that the through-put of the clinic can be greatly
increased." [F2]
She explains that Ambulight is effective and addresses the problem of
pain in conventional PDT. For example she comments on a recent study
conducted in which "we have reported on 53 patients with 61 lesions
...who were treated with Ambulight PDT and, again, pain scores were low,
but importantly efficacy at one year follow-up was high with 84% of
lesions being clear." [F2]
She adds "Thus to summarise, Ambulight PDT is extremely convenient,
easy to use, associated with low levels of discomfort and is highly
effective for the treatment of these superficial non-melanoma skin
cancers and dysplasia. The use of these devices allows greater
through-put and efficiency of the PDT clinic and thus has major benefits
both for the patients, allowing them a comfortable, portable, effective
home-based treatment, but also for the running of the PDT clinic. The
treatment procedure is simplified and the number of patients that can be
treated in any given clinic session increased. With regard to the bigger
picture, Ambulight PDT certainly enhances the wider acceptance of PDT in
the community as pain had been a limiting factor for some referrers to
the service. Ambulight PDT now has a very important role in our own PDT
services and my understanding is that it has now been taken up in many
centres. Feedback indicated that 27 centres in the Netherlands are now
using Ambulight PDT, and uptake is ongoing in the UK. Thus, it has made
a significant impact in terms of change of practice in how we deliver
PDT." [F2]
The Principal Scientist at Ambicare Health has also written confirming
the above points about Ambulight Multi [F1]. He also explains the Lustre
product: "Lustre is a wearable light source for acne treatment with
blue light. Acne has a major impact on the lives of many sufferers, and
in a clinical trial we have shown that Lustre offers major advantages in
reducing lesions. The advantage of Lustre is that it enables acne to be
treated in the comfort of the home, and without drugs or chemicals."
He adds "Blue light therapy for the treatment of acne has been around
for many years, however existing treatment typically come in one of two
forms; torches that are held to the face by the user, or lamps that
shine remotely onto the skin. Both are highly inconvenient, particularly
in a home setting and consequently users are highly unmotivated to use
them. This leads to low treatment compliance and ultimately poor
efficacy. Lustre in contrast, is a light weight wearable device that
allows users to get on with their lives; this ease of use promotes
compliance and ultimately efficacy. This is very appealing to acne
sufferers and doctors treating them. At present Lustre is being sold
mainly in the Netherlands and the UK. It is in regular use in over 250
clinics in the UK." [F1]
He also comments "In 2012 the Lustre Pure Light device won the
Aesthetic Industry Awards, Product innovation of the year." [F1]
Sources to corroborate the impact