Protecting Women from HIV AIDS: Dapivirine Vaginal Ring HIV Microbicide
Submitting Institution
Queen's University BelfastUnit of Assessment
Allied Health Professions, Dentistry, Nursing and PharmacySummary Impact Type
TechnologicalResearch Subject Area(s)
Medical and Health Sciences: Clinical Sciences, Medical Microbiology, Public Health and Health Services
Summary of the impact
In sub-Saharan Africa, 22 million people live with HIV/AIDS. Annual
mortality is 1.5 million and
sexual transmission accounts for ~90% of new infections. Young women are
disproportionately
affected due to socio-cultural issues. Seeking to empower them with an
urgently needed female-initiated
protective method, Malcolm & Woolfson developed the first
antiretroviral (AR) microbicide
vaginal ring (VR), which provides slow, continuous release of dapivirine
for long-lasting protection
against vaginal HIV transmission. Consequently, global microbicide
development strategies were
transformed, with the focus shifted from immediate-use gels to long-acting
VRs. In August 2012,
the dapivirine VR commenced final stage (Phase III) clinical trials in
Africa.
Underpinning research
Woolfson1 (Chair in Pharmaceutics, School of Pharmacy
QUB) contributed substantially to the
development of a hormone replacement therapy VR product, marketed as
Femring® in the USA by
Warner Chilcott Inc., that provides controlled release (CR) of estrogen
for three months from a
single device. The ability of VR devices to provide long-term continuous
drug release for up to one
year, coupled with their ease of user insertion and removal, make them
highly relevant to the field
of HIV microbicides. In this context, microbicides are defined as
compounds applied inside the
vagina to protect against sexually transmitted infections (STIs),
including HIV. In the continued
absence of a viable HIV vaccine, development of a vaginal microbicide is
widely acknowledged to
be the most practical strategy for the prevention of sexual HIV
transmission. Early microbicide
formulation strategies focused on gel products, intended for
administration a short time before
each act of intercourse. However, these gel-based microbicide strategies
continue to be hindered
by low user acceptability and poor adherance, particularly in the
developing world. By contrast,
VRs overcome many of these obstacles, since they can (i) be used without
the knowledge of the
male partner, (ii) be worn continuously, thereby offering
`round-the-clock' protection, and (iii) offer
significantly increased levels of user compliance and acceptability.
Malcolm (Chair in Drug Delivery, School of Pharmacy QUB) and Woolfson
published the first
study2 describing release of a candidate microbicide
from a VR in 2003, along with fundamental
research describing injection moulding of VRs and drug release mechanisms
3-4. These studies
established predictive models for determining the optimal physicochemical
characteristics
(hydrophobicity, molecular weight, etc.) of drugs for effective release
from VRs, enabling VR
technology to be applied to newer AR compounds. Since then, Malcolm and
Woolfson have
published many further papers on this subject, plus several book
chapters/reviews on VR delivery
technologies and their potential application to HIV microbicide
development.
With the clinical failure of early non-specific microbicide candidates,
the International Partnership
for Microbicides (IPM) was established in Washington DC as an
international development agency
to lead efforts in delivering an effective microbicide product. With
financial backing from major
governments in the developed world and other leading global health
organisations, such as UK
DFID, The Bill and Melinda Gates Foundation, and The Rockefeller
Foundation, IPM focused
attention on CR delivery of potent AR drugs. Malcolm and Woolfson received
funding from IPM to
develop a VR device releasing the non-nucleoside reverse transcriptase
inhibitor (NNRTI)
dapivirine (also referred to as TMC 120). The key papers resulting from
this work were published in
20055 and, in more detail, late 20066.
IPM funding has continued to date, now totalling £1.8M and
further supplemented by three major EU Framework programmes and NIH
funding. This funding
has established two dedicated IPM laboratories in the School of Pharmacy
QUB for the
development, manufacture and testing of microbicide VRs.
References to the research
1. Woolfson, A. D., Elliott, G.R.E., Gilligan, Claire A.
and Passmore, Clare M. Design of an
intravaginal ring for the controlled delivery of 17beta-estradiol as its
3-acetate ester. Journal of
Controlled Release 61. 319-328. 1999.
2. Malcolm K.; Woolfson D.; Russell J.; Andrews C. In vitro
release of nonoxynol-9 from silicone
matrix intravaginal rings Journal of Controlled Release 91.
355-364. 2003.
3. Malcolm R.K, Woolfson A.D., Russell J.A. Tallon, P.,
McAuley L,. Craig D.Q.M. Influence of
silicone elastomer solubility and diffusivity on the in-vitro release of
drugs from intravaginal rings.
Journal of Controlled Release 90. 217-225. 2003.
4. Malcolm, RK, McCullagh, S, Woolfson, AD, M. Catney, M,
Tallon, P. A dynamic mechanical
method for determining the silicone elastomer solubility of drugs and
pharmaceutical excipients in
silicone intravaginal drug delivery rings. Biomaterials. 23.
3589-3594. 2002.
5. Malcolm, R. K., Woolfson, A. D., Toner, C. F., Morrow,
R. J. and McCullagh, S. D. Long-term,
controlled release of the HIV microbicide TMC120 from silicone elastomer
vaginal rings. Journal of
Antimicrobial Chemotherapy 56. 954-956. 2005.
6. Woolfson, A.D., Malcolm, R.K., Morrow, R.J., Toner,
C.F., McCullagh, S.D. Intravaginal Ring
Delivery of the Reverse Transcriptase Inhibitor TMC 120 as an HIV
Microbicide. International
Journal of Pharmaceutics 325. 82-89. 2006.
Research Grants (Malcom and Woolfson): Development of vaginal ring
microbicides.
International Partnership for Microbicides, Washington DC. £1.8M (four
grants from 2004 -20012);
EUFP7 Consortium on Combined HIV Microbicide Delivery (CHAARM), £263k (to
QUB), 2010 -14
and EUFP6 European Microbicides Project (EMPRO), £122k (to QUB), 2004-8;
NIH U19/Cornell
Univ, USA, Vaginal microbicides, £370k to QUB, 2007-12.
Details of the impact
In this case study, impact is seen in (i) transfer of the research
from the laboratory to a
global sponsor, (ii) a major shift in the sponsor's policy priorities
for microbicide delivery
(moving to a controlled release VR system that is coitally independent
and away from
immediate release, coitally dependent gel products), (iii) investment in
successful Phase I
and II trials, and a multi-million dollar investment in Phase III by
both IPM and the US
government (NIH), and (iv) use of the product by thousands of women
enrolling in the trials
for protection against HIV infection.
HIV/AIDS is the leading cause of death for women aged 15-44 worldwide,
with most deaths
occurring in sub-Saharan Africa. For physiological reasons, women are
twice as likely than men to
contract HIV from a single act of unprotected sex. However, societal and
cultural prejudices mean
that women are often highly dependent on male cooperation to protect
themselves from infection.
Compared with other female-initiated microbicide strategies, vaginal ring
products are widely acknowledged to offer the greatest potential. Their
ability
to be used covertly without the knowledge or co-operation of the male
partner, the relatively high user acceptabilitya and the
expectation of
increased user adherenceb in long-term use schedules
suggest that a
microbicide-releasing ring against HIV will challenge existing sexual
norms
within many developing world cultures by empowering women to take control
of their own sexual health. Malcolm and Woolfson's work on VR microbicide
delivery will provide women, for the first time, with a means of
protecting
themselves from heterosexually acquired HIV infection, without requiring
support from the male partner. This is reflected in the change of
direction from the key microbicide
development agency, IPM, where £millions are now being invested in
advanced trials (Phase III) of
the first microbicide VR, developed by Malcolm and Woolfson, as a result
of the impact of their
technology on the microbicide field.

Following initial failings with the first generation non-specific
anti-HIV compounds, microbicide
research became focused on potent small molecule ARs similar to those used
orally in Highly
Active Anti-Retroviral Therapy (HAART). With this approach, a VR device
must continuously
deliver an AR compound into the vaginal tissue over an extended time
period in
order to provide long-term protection. This required the pre-clinical
development of an appropriate VR by Malcolm and Woolfson showing product
performance characteristics (drug release, stability, durability, ease of
manufacture) sufficient to justify the substantial financial commitment to
take a
product through clinical trials.

Malcolm and Woolfson served on IPM's first Scientific Advisory Board in
2004c
and the group is currently listed as IPM's VR microbicide development
partnerd.
The dapivirine-releasing VR developed by Malcolm and Woolfson has been
adopted by IPM as its
lead product, on the basis of its sustained drug release kinetics and
excellent safety profile. IPM
and its partners are funding a
full clinical trials programmee
for the dapivirine VR, as
detailed below. Since 2008f,g,
three Phase I safety and
availability clinical trials, IPM
001, 008 and 013 have been
completed in Belgium. Also
completed are a series of
Phase II trials in HIV negative
women. Trials IPM 001, 008
and 018 determined dapivirine
concentrations in plasma and
vaginal fluid samples, with
safety assessed by pelvic/
colposcopic examinations,
clinical laboratory tests, and
adverse events. VRs were well
tolerated with similar adverse
events observed in the placebo
and dapivirine groups.
Dapivirine was successfully distributed throughout the lower genital
tract at concentrations over 4
logs greater than the EC50 against wild-type HIV-1 (LAI) in MT4 cells.
Mean plasma
concentrations of dapivirine were < 50 pg/ml, an important observation
since high systemic drug
levels are undesirable in a vaginal microbicide due to the potential for
development of resistance to
the virus in infected users. Based on these successful trials and
related safety studies, the
dapivirine VR developed by Malcolm and Woolfson has now entered into two
major, multi-centre
pivotal Phase III trials in Africa, which commenced in August 2012.
These trials
(described below) involve thousands of women In using the dapivirine VR
as protection
against heterosexually acquired HIV AIDS.
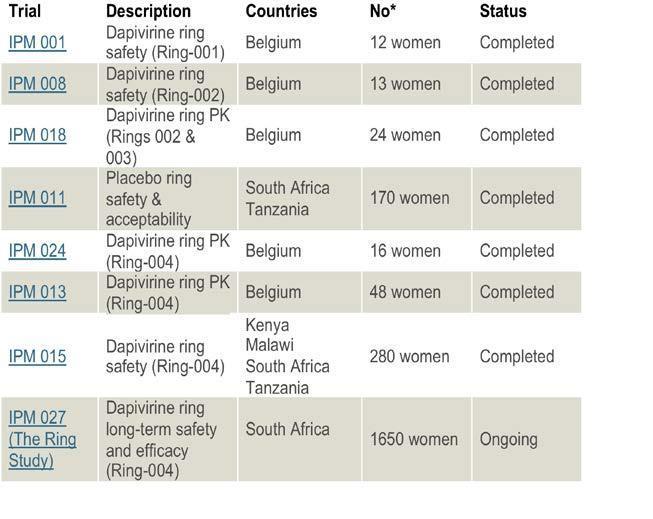
ASPIRE, also known as MTN-020, is a Phase III clinical study
funded by the US National Institutes
of Health through the Microbicides Trial Network (MTN). It seeks to
determine safety and efficacy
of the dapivirine VR for protecting against the sexual transmission of HIV
when used by women for a month at a time. The study, which has started to
enrol 3,476 women across several sites in Africa, will take approximately
two
years to conduct. Simultaneously, IPM's pivotal Phase III RING STUDY
(IPM
027) is enrolling 1650 women. IPM have published a detailed `Access
Strategy' (May 2011)h for licensing andworldwide
availability of the dapivirine
VR, with these pivotal trials being key to final product registration.
This trial
information, involving substantial investment by IPM, demonstrates the
outcome of the pre-clinical dapivirine VR research reported in 2005 and
2006 by Malcolm and
Woolfsoni, j.

Sources to corroborate the impact
VR Acceptability studies
(a) Hardy E, Hebling EM, Sousa MH, Almeida AF, Amaral E, Delivery
of microbicides to the
vagina: difficulties reported with the use of three devices, adherence to
use and preferences,
Contraception 76.126-131. 2007. (DOI:
10.1016/j.contraception.2007.04.013)
(b) Ahrendt HJ, Nisand I, Bastianelli C, Gomez MA,
Gemzell-Danielsson K, Urdl W, Karskov B,
Oeyen L, Bitzer J, Page G, Milsom I. Efficacy, acceptability and
tolerability of the combined
contraceptive ring, NuvaRing, compared with an oral contraceptive
containing 30 mu g of ethinyl
estradiol and 3 mg of drospirenone Contraception 74. 451-457. 206.
(DOI: 10.1016/j.contraception.2006.07.004)
Malcolm and Woolfson: founding members of IPM's Scientific Advisory
Board
(c) http://www.ipmglobal.org/publications/2004-ipm-annual-report
page 23
QUB link to IPM as VR product development partner
(d) www.ipmglobal.org/our-partners/product-development-partners
Clinical trials on dapivirine VR microbicide
(e) www.ipmglobal.org/our-work/research/clinical-trial.
Paper (2009) detailing results from IPM 018 safety and pharmacokinetic
study on QUB VR
dapivirine ring, transferred to IPM for clinical development — paper
links directly to References 5
and 6, Section 3.
(f) Nel A, Smythe S, Young K, Malcolm K, McCoy C, Rosenberg Z,
Romano J . Safety and
Pharmacokinetics of Dapivirine Delivery From Matrix and Reservoir
Intravaginal Rings to HIV-Negative
Women. JAIDS-Journal of Acquired Immune Deficiency Syndromes 51.
416-423. 2009.
Paper presenting data from Phase 1 trials IPM 001 and 008
(g) Romano J, Variano B, Coplan P, Van Roey J, Douville K,
Temmerman M, Verstraelen H, Van
Bortel L, Weyers S, Mitchnick M. Safety and Availability of Dapivirine
(TMC120) Delivered from an
Intravaginal Ring. AIDS Research and Human Retroviruses 25.
483-488. 2009.
(DOI: 10.1089/aid.2008.0184)
Global access strategy for dapivirine VR
(h) http://www.ipmglobal.org/publications/preparing-access-microbicides-and-dapivirine-ring-hiv-prevention-preliminary-strategy
(i) Independent corroboration of the development of dapivirine
VR by Malcolm and Woolfson and
its subsequent clinical trial history
Chair, Research and Advisory Steering Committee, International Partnership
for Microbicides,
Washington DC.
(j) Platinum-catalyzed intravaginal rings. US Patent Application
20120093911 claiming priority to
US Provisional Application Serial No. 61/394,493, naming Malcolm and
Woolfson as inventors of
the dapivirine intravaginal ring, patent rights assigned by Queen's
University to IPM to IPM.