Commercial products for improved oral health based upon novel antioxidant micronutrient approaches delivered via toothpastes and food capsules
Submitting Institution
University of BirminghamUnit of Assessment
Allied Health Professions, Dentistry, Nursing and PharmacySummary Impact Type
TechnologicalResearch Subject Area(s)
Medical and Health Sciences: Clinical Sciences, Dentistry
Summary of the impact
Pioneering basic research into the role of oxygen free- radical damage
and antioxidant micro-nutrient protection in human periodontal diseases by
the Periodontal Research Group in Birmingham has led to the development
and marketing of novel toothpaste formulations and new applications for
other nutrient products in collaboration with global consumer healthcare
companies. This work has changed thinking in the field and has had
significant commercial impact in terms of changing business
R&D and marketing strategies. Resultant technologies have
demonstrated reductions in gingivitis and periodontitis with associated social,
economic and health impacts. In addition, our research is enabling
Triclosan, an antibacterial compound used widely in soaps, detergents,
mouthwashes and toothpastes, to be replaced with more
environmentally-friendly, natural and equally efficacious agents.
Underpinning research
Periodontitis is a severe form of gum disease (periodontal disease) and
the most common chronic inflammatory disease of humans, affecting 50% of
adults globally. Whilst it is initiated by the accumulation of a bacterial
plaque biofilm at and below the gum margin, 80% of the resultant tissue
damage is caused by an exaggerated host immune response to the plaque
biofilm. Whilst plaque removal by tooth brushing is key to prevention and
treatment, therapeutic outcomes are limited and methods of modulating the
hosts' immune response are needed. Not only is periodontitis a major cause
of tooth loss worldwide it is also a significant independent risk factor
for atherogenic cardiovascular disease and diabetes, due to bacterial
entry into the bloodstream and the resultant acute-phase response and
oxidative stress that ensues.
The Periodontal Research Group led by Professor Iain Chapple and Dr John
Matthews (Reader; 1993-current) and involving Drs Melissa Grant (Former
Research Fellow, Lecturer; 2011-current) and Michael Milward (Senior
Lecturer; 2005-current) has pioneered research into antioxidant and free
radical biology and its impact on chronic inflammatory diseases
(particularly periodontal diseases) since 1996. Antioxidant micronutrients
are natural dietary compounds that protect the body's cells and tissues
from excessive release of damaging oxygen radicals, preventing oxidative
stress and therefore the generation of destructive inflammation that
characterises periodontitis. They act at atomic and molecular levels via
cell signalling cascades. Our work in this field began with the
development of an enhanced chemiluminescence assay to measure small
molecule total antioxidant capacity in biological fluids and tissues (1)
and led to the elucidation of reduced glutathione (GSH) as the key
antioxidant at exposed epithelial surfaces, which was deficient in both
periodontal and lung disease (2, 3).
A hypothesis that GSH was key in regulating inflammation via NF03baB
modulation within mucosal tissues was proposed by us in 1996 (Chapple et
al J Clin Molec Pathol 1996:49;M247-55; 2nd most read
paper in journal/year) and 1997 (Chapple et al J Clin Perio
1997:24;287-96: 189 citations), identifying NF03baB antagonists as
potential therapeutic targets. This thesis has since been proven (3) and
several therapeutic strategies using GSH have been adopted in medicine for
inflammatory disease management. In addition, targeting the NF03baB
pathway using natural and pharmacological approaches has become a major
focus in the development of anti-inflammatory therapies in general. Our
approach aims to facilitate GSH preservation by boosting tissue
antioxidant status through either topical application in toothpastes or
systemic application via a parenteral route using micronutrient capsules,
and to stimulate GSH synthesis by activation of the anti-inflammatory
transcription factor Nrf2 [Nuclear factor (erythroid-derived 2)-like 2]
(3).
Large scale epidemiological studies (4) and case-control studies (2004)
confirmed antioxidant micronutrient deficiency in periodontitis and an
intervention study (Chapple et al J Clin Perio 2007:34; 103-10)
demonstrated that resolving periodontal inflammation led to antioxidant
recovery. In parallel with work on antioxidant biology, studies
investigating the origins of oxidative stress in peripheral blood
neutrophils from periodontitis patients (3,5,6) demonstrated these cells
as being dysregulated in periodontitis patients and identified mechanisms,
which have subsequently formed a target for novel toothpaste formulations
(3,6). Studies on oral epithelial cells also demonstrated that NF03baB
modulation was possible using micronutritional approaches (7). This,
alongside work on neutrophil biology, led to translational studies aimed
at developing local and systemic approaches for modulating periodontal
inflammation in collaboration with industry, leading to product
development (3,5,6).
This research has resulted in keynote lectures/symposia at the
International Association for Dental Research (2005, 2009, 2011, 2012);
the British Society for Oral and Dental Research (2004, 2013); the
European Federation of Periodontology (2003, 2006, 2009, 2012); American
Nutraceutical Association (2010); Royal Society of Medicine (2010); and
several awards: Australian Dental Association Eminent Lectureship 2010;
The Charles Tomes Medal and Lecture, The Royal College of Surgeons England
2010; International lectures for national periodontal societies in
Germany, Belgium, Switzerland, Holland, Denmark, Spain, Mexico, Greece
(2010-2013). The lectures have focussed on how we have improved
understanding of the oxidative stress process and its impact in
periodontitis and as a result, reported novel therapeutic approaches.
References to the research
1. Chapple ILC, Mason GM, Matthews JB et al. Enhanced
chemiluminescent assay for measuring the total antioxidant capacity of
serum, saliva and crevicular fluid. Ann Clin Biochem. 1997: 34;412-421.
doi: 10.1177/000456329703400413.
2. Chapple ILC, Brock G, Eftimiadi C, et al. Glutathione in
gingival crevicular fluid and its relation to local antioxidant capacity
in periodontal health and disease. Mol Path, 2002:78,55,367-373.
doi:10.1136/mp.55.6.367.
3. Dias HK, Chapple ILC, Milward MR et al. Sulforaphane restores
cellular glutathione level and reduces chronic periodontitis neutrophil
hyperactivity in vitro. PLoS ONE. 2013:8(6):e66407.
doi:10.1371/journal.pone.0066407.
4. Chapple ILC, Milward M, Dietrich T. The prevalence of inflammatory
periodontitis is negatively associated with serum antioxidant
concentrations J Nutr. 2007:137;657-64.
5. Matthews JB, Wright H, Roberts A et al. Hyperactivity and
reactivity of peripheral blood neutrophils in chronic periodontitis. Clin
Exp Immunol, 2007:147;255-64. doi: 10.1111/j.1365-2249.2006.03276.x.
6. Chapple ILC, Matthews JB, Wright HJ et al. Ascorbate and
α-tocopherol differentially modulate reactive oxygen species generation by
neutrophils in response to Fc03b3R and TLR agonists. Innate Immunity
2013:19;15-9. doi: 10.1177/1753425912455207.
7. Milward MR, Chapple ILC, Grant MM et al. The action of a
natural antioxidant on periodontal pathogen stimulated oral keratinocytes.
Innate Immunity 2013:19;140-51. doi: 10.1177/1753425912454761.
Details of the impact
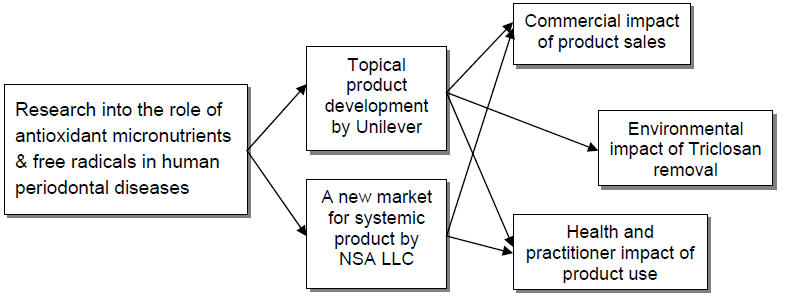
A. Commercial Impact
Topical product development by Unilever
With more than 50% of the adult UK population affected by periodontal
disease, the cost to the UK economy alone was estimated at £2.78-billion
in 2008 (ATP Consulting 2008). As such, novel treatment modalities for
modulating periodontal inflammation represent an enormous market for
healthcare companies. Unilever, a global consumer healthcare company, has
invested several million GBP in research & development (R&D)
activities (£1.5-million directly to the University of Birmingham's
Periodontal Research Group) based on our investigator-led collaborative
proposals. Our work has defined mechanisms by which oxidative stress
drives inflammation in different model systems and we subsequently
developed a Platform Evaluation Capability (PEC1 & PEC2 programs) to
enable the high-throughput screening of natural micronutrients for
anti-inflammatory properties (e1, e2, e3). We have investigated the
highest-performing new actives in our model systems and elucidated their
mechanisms of action. This research has resulted in the development of a
new health product concept of micronutritional approaches for modulating
inflammation, its introduction to a global market, and further development
of combined actives to secure intellectual property and patents. Staff
across Unilever's R&D centres are actively involved in exploiting the
data from ongoing studies, with the novel insights and science being used
in the identification of new technologies and next-generation market
innovations.
A new clinical intervention has been developed from basic research in
collaboration with Unilever PLC (70% Birmingham), which has transformed
traditional approaches to combating gingivitis and periodontitis, and is
delivered topically through a toothpaste formulation.
1. Proof-of-principle phase-1 product was developed and taken to market
in 2008 in a low profile approach to assess market uptake and introduce a
new therapeutic concept based upon oral tissue nourishment (Unilever
NutriActiv toothpaste — e1). This was launched in many of the key and
largest European oral care markets, including France, Italy, Greece and
central Europe (e2).
2. Pivotal phase-2 studies have been run with a product that employs a
combination of 3 micro-nutrients and a key clinical study is currently
underway with our group (May-Nov 2013), with a view to a new product
launch in 2014/15. The study outcomes are commercially sensitive but will
be published as a journal supplement for claims support, similar to that
under (e1).
Systemic product application and new market development by NSA LLC
The application of an existing nutritional intervention has also been
expanded to a new global market through an investigator-led Randomised
Controlled Trial (2012), the first of its kind in oral care research.
Based upon our ideas and research, National Safety Associates (NSA LLC,
Memphis), a US-based company who make a product known as "JuicePlus®",
has funded 3 investigator-led studies. The studies are aimed at
elucidating the clinical benefits of JuicePlus®, a
phytonutrient dietary intervention (in capsule form), as an adjunct to
periodontal therapy (clinicaltrials.gov NCT00952536), as a
mono-therapy (multi-centre, 3-country study underway — clinicaltrials.gov
NCT01229631) and also as an approach for reducing post-operative morbidity
and improving healing following wisdom tooth surgery (clinicaltrials.gov
NCT01145820).
Successful outcomes from the first study are already
published, creating a new use for the product and a new market (oral
healthcare) for the company. This has opened up an entirely novel and
substantial business opportunity for NSA (e3) with data from the first
study being disseminated globally via the literature, and in academic and
business conferences in the USA, Europe and the UK. This has assisted NSA
in the planning and marketing of their global business, which generates
over US$350-million in sales annually (>0.5-million customers in the
USA alone) across more than 20 countries around the world, providing
global reach for such oral health benefits (e3).
Wider market recognition and adoption within healthcare industry
Johnson and Johnson (J&J) have now recently engaged our group in
analyzing, using our in-house assays, the antioxidant capacity of their
mouthrinse formulations (January 2012 — current), and are planning product
revisions and developments based on these outcomes (e3). This research has
highlighted to J&J the significant potential of mouthrinses in
contributing to oral health through previously unexplored properties.
Consequently, J&J have invested, to-date, in excess of £100k in
support of R&D activities in this area in partnership with us.
B. Health and Practitioner Impacts
Our research on
antioxidant micronutrients and their demonstrable modulation of innate
immune responses has contributed to an entirely new therapeutic field in
Dentistry and has been recognized globally. Several countries, consortia
of countries through European and International academic associations, and
European bodies have engaged in symposia, workshops and have published
consensus statements from expert groups on the importance of nutritional
advice for periodontal care (e.g. 7th European Workshop on Periodontology
2010 (e4), the leading influence in the discipline in Europe and now the
world). This document highlights the need to engage patients in practice
with nutritional advice in the management of their periodontal disease.
Our paper in J Clin Perio (e5) demonstrated periodontal health
benefits from micronutrient capsules and was immediately identified by the
editor of the top impact factor dental journal in the world for a pubcast,
and made `open access', in order to facilitate public and practitioner
dissemination. The message has also reached the world's Integrative
Medicine community, by a published interview on the paper in a US-based
journal, invited within 21-days of the paper's release (e6). Nationally,
industry-funded road-shows have disseminated these findings (2011-2012) to
dental care professionals (over 2000 delegates across 10 UK venues), and
stimulated substantial interest, with evidence of changes in practice,
provided by feedback from practitioner delegates (e7). A chapter by
Chapple and Grant was also commissioned in a new textbook Food
constituents and oral health (Woodhead Publishing LTD., Chapter 11,
ISBN:978-1-84569-153-0) which is targeted at educating practitioners in
these new adjunctive treatment modalities. Our micronutrient results have
also been cascaded to the global integrative medicine community (e8).
C. Environmental Impactm
Our novel toothpaste actives, developed in collaboration with Unilever,
provide a viable alternative to the use of Triclosan, a chlorinated
aromatic antibacterial compound used widely in soaps, detergents,
mouthwashes and toothpastes. Triclosan is toxic to aquatic bacteria at
levels found in the environment and inhibits photosynthesis in key algae
responsible for a large part of the photosynthesis which occurs on earth.
To be able to replace Triclosan with natural active ingredients that
provide equivalence in clinical efficacy, without adverse effects upon the
environment, is a longer-term goal for companies such as Unilever. While
this will depend on local product affordability in different countries in
the short term, our phase 2 data now provides proof of principle that this
is an achievable goal using micronutrient approaches and longer-term this
will likely lower the costs of oral healthcare products, targeting
universal affordability (e2, e3).
Sources to corroborate the impact
e1. An entire supplement of the International Dental Journal, sponsored
by Unilever, was devoted to healthcare benefits from the administration of
micronutrients within a toothpaste formulation. International Dent Journal
2007: 57; S2; 117-149. Available upon request.
e2. Unilever letter outlining impact on strategic direction of oral care
business, market reach of Unilever, sales data on NutiActiv, expected
sales data on phase-2 formulation.
e3. Confirmation that services and policies of 3 major multi-national
companies have been influenced and driven by our research in this field,
specifically Unilever, NSA and J&J. Impact upon national societies and
professional opinion is evidenced by invited lectures.
e4. The European Federation of Periodontology have also published via
open access a keynote review paper and consensus report for the profession
and public (J Clin Periodontol 2011:38;suppl 11:114-118. doi:
10.1111/j.1600-051X.2010.01675.x; J Clin Periodontol 2011: 38; suppl 11:
142-158. doi: 10.1111/j.1600-051X.2010.01663.x.).
e5. Chapple ILC, Milward MR, Ling-Mountford N, Weston P, Carter K, Askey
K, Dallal GE, De Spirt S, Sies H, Patel D and Matthews JB. (2012),
Adjunctive daily supplementation with encapsulated fruit, vegetable and
berry juice powder concentrates and clinical periodontal outcomes: a
double-blind RCT. J of Clin Periodontol, 39: 62-72. doi:
10.1111/j.1600-051X.2011.01793.x. Only open access paper in volume.
e6. Record of interview with US-based Integrative Medicine journal (www.vitasearch.com).
e7. Letter from AB Communications confirming feedback from P&G
lecture series 2011-12.
e8. Evidence of clinical benefit also from presentations at Experimental
Biology (Anaheim 24th March 2010); The American Nutraceutical Academy
(ANA) (Pheonix 2010, 25th March 2010); The ANA (Palm Beach California 24th
April 2011); The European Congress on Integrative Medicine (ENA — Berlin —
4th December 2010).