UoA01-15: Accurate Diagnosis: Improving Survival Rates for Children with Cancer
Submitting Institution
University of OxfordUnit of Assessment
Clinical MedicineSummary Impact Type
HealthResearch Subject Area(s)
Medical and Health Sciences: Immunology, Oncology and Carcinogenesis
Summary of the impact
The production and use of monoclonal antibody, ALK1, by researchers in
Oxford has been pivotal in enabling the accurate diagnosis and treatment
of Anaplastic Large Cell Lymphoma (ALCL). This research also led to the
formal classification of ALK-positive ALCL tumours by the World Health
Organization in 2008. While ALCL accounts for 10-20% of
paediatric/adolescent non-Hodgkin's lymphoma worldwide, its diagnosis had
been problematical due to the absence of suitable reagents. This was
remedied in 1997 when Oxford researchers created the first monoclonal
antibody, ALK1, recognising anaplastic lymphoma kinase (ALK), a molecule
that is associated with up to 90% of ALCL.
Underpinning research
Before 1997 Anaplastic large cell lymphoma (ALCL) posed a major
diagnostic problem to clinicians because of the lack of reagents able to
distinguish ALCL from other tumours. Patients were frequently misdiagnosed
with carcinoma, histiocytosis X or Hodgkin's disease, leading to
unnecessary and often invasive therapy, including surgery. In 1997 the
late Professor David Y. Mason (deceased Feb 2008), Dr Karen Pulford, and
the Leukaemia Research Fund Immunodiagnostics Unit (now Leukaemia and
Lymphoma Research Fund) produced the first monoclonal antibody, ALK1,
against the anaplastic lymphoma kinase (ALK) protein, which is the
principal cause of oncogenesis in ALCL1. Oncogenic
translocations create fusion proteins of ALK and partners capable of high
expression and dimerization, after which dimerisation leads to ALK
autophosphorylation and constitutive activation.
The ALK1 antibody made a precise diagnosis of ALK-positive ALCL possible
for the first time. The ALK1 antibody identified ALK-positive ALCL as a
molecular pathological entity (distinct from ALK-negative ALCL), showing
that this cancer accounts for 10-20% of childhood lymphomas and 3% of
adult non-Hodgkins lymphomas2. Due to improved survival rates
associated with ALK-positive ALCL, the ability to distinguish between
ALK-positive and negative forms of the disease represented a vitally
important step in achieving accurate diagnosis and appropriate treatment
for patients.
Researchers at the University of Oxford have gone on to use the antibody
ALK1 to identify additional ALK fusion proteins in ALCL and confirm the
role of the ALK proteins play a primary role in tumour development 3.
They have shown that ALK fusion proteins may be immunogenic and candidates
for immunotherapy4.
The antibody ALK1 has been used to show ALK protein expression in
neuroblastoma5, and ALK has also been identified in a number of
other solid tumours, such as lung cancer. Studies on the immune response
to ALK, using ALK1 antibody as an essential reagent (initiated by Oxford
and later performed as collaborative studies within international phase
III clinical trials6), are included in the clinical trial `ALCL
2012'. This phase III clinical study organised by the European Inter-group
for Childhood non-Hodgkin's Lymphoma (EICNHL) will identify high-risk
patients so that they can be directed to more effective therapies as soon
as possible.
References to the research
1. Pulford, K. et al. Detection of anaplastic lymphoma
kinase (ALK) and nucleolar protein nucleophosmin (NPM)-ALK proteins in
normal and neoplastic cells with the monoclonal antibody ALK1. Blood
89, 1394-404 (1997). Describes the production and
characterization of the monoclonal antibody ALK1.
2. Stein, H. et al. CD30(+) anaplastic large cell lymphoma: a
review of its histopathologic, genetic, and clinical features. Blood
96, 3681-95 (2000). Collaborative review of the work leading
up to description of ALK-positive ALCL
3. Bischoff, D., Pulford, K., Mason, D.Y., & Morris, S.W. Role of the
nucleophosmin (NPM) portion of the non-Hodgkin's lymphoma-associated
NPM-anaplastic lymphoma kinase fusion protein in oncogenesis. Mol Cell
Biol. 17, 2312-2325 (1997). Reference to the importance of
the NPM-ALK fusion protein.
4. Ait-Tahar, K., et al. Correlation of the autoantibody response
to the ALK oncoantigen in pediatric anaplastic lymphoma kinase-positive
anaplastic large cell lymphoma with tumor dissemination and relapse risk.
Blood 115, 3314-9 (2010). doi:
10.1182/blood-2009-11-251892. ª From Oxford and *Joint last authors. Description
of the immunogenicity of ALK protein and the first description of the
immune response to ALK being of prognostic significance and also
having a potential role in tumour spread.
5. Lamant, L. et al. Expression of the ALK tyrosine kinase gene
in neuroblastoma. Am J Pathol 156, 1711-21 (2000). First
description of ALK being expressed in neuroblastoma.
6. National Cancer Institute (NCI). COG-ANHL0131 - Phase III Randomized
Study of Consolidation Chemotherapy Comprising Doxorubicin and Prednisone
in Combination With Vincristine Versus Vinblastine in Patients With
Advanced Anaplastic Large Cell Lymphoma. In ClinicalTrials.gov [Internet].
Bathesda (MD): National Library of Medicine (US). 2000-[cited 2013 Apr
04]. Available from: http://clinicaltrials.gov/show/NCT00059839
NLM Identifier: NCT00059839. Clinical trial on ALCL in which the
biological studies on the immune response to ALK involving the ALK1
antibody was studied.
This research was funded by the Leukaemia and Lymphoma Research Fund,
Cancer Research UK, the Starmer-Smith Memorial Fund, Sam Foye Fund and the
Medical Research Fund of the University of Oxford.
Details of the impact
The production and use of the antibody ALK1 by the University of Oxford
has had a major impact on lymphoma diagnosis and provided invaluable
information on tumour development. ALK-positive ALCL is now also included
in the World Health Organization's (WHO) current classification of
haematological malignancies.
Accurate Diagnosis
The production and use of the antibody ALK1 by the University of Oxford
has had a major impact on lymphoma diagnosis, enabling the definitive
diagnosis of the tumour entity ALK-positive ALCL. ALCL was previously
regarded as an aggressive incurable disease and frequently misdiagnosed as
a carcinoma or other haematological malignancy, resulting in inappropriate
treatment. Not only has the antibody ALK1 revolutionised the accurate
diagnosis and understanding of ALK-positive ALCL, this tumour now
represents the best characterised T-cell lymphoma, with the exception of
cutaneous T-cell lymphoma, which has a far worse prognosis7,8,9.
Importantly, the sensitivity of the antibody has permitted the detection
of minimal residual disease. The latter is an important factor in cancers
since it can lead to a failure to detect disease and result in the patient
relapsing. A vital element of the value of an antibody for diagnostic use
is its inclusion in the NEQAS scheme. The United Kingdom National External
Quality Assessment Service (UK NEQAS) ensures the accuracy and reliability
of laboratory tests and is used by all diagnostic labs. ALK1 fulfills this
category. The following statement was received via email on the 19th
of September 2012, from Doctor Merdol Ibrahim, Manager of UK NEQAS-ICC.
This email, and the antibody usage table, has been kept on file: "NEQAS
requested ALK for its lymphoma module about 1.5 years ago for the first
time. We distributed a composite control of a tonsil and anaplastic
large cell lymphoma for participants to stain. Participants also
submitted their methodologies and I have attached the antibody usage
table (second table on the right), which shows that 104/179 (58%)
participants used the Dako CD246 clone. It is one of the most popular
antibodies and had a very good pass rate with respect to the expected
staining levels."10
Policy and Guidelines
The identification of ALK-positive ALCL (as opposed to ALK-negative ALCL)
has gained worldwide acceptance and is now included in the current
classification of haematological malignancies, first published by the
World Health Organization in 2008. ALK1 is considered to be the gold
standard antibody for the diagnosis of ALK-positive ALCL9.
Clinical outcomes
Approximately 870 children are diagnosed with non-Hodgkin's lymphoma every
year in the USA 11, equating to about 175 new cases of ALK+ALCL
annually. For adults, this figure rises to approximately 1,50012.
Before 1997 it was difficult to compare the survival rates of patients
with ALCL due to problems with the actual diagnosis of the disease. This
was compounded by a lack of common staging systems, relatively small
numbers of patients, and a variety of different treatment regimens being
used in the clinics. This meant that patients may have undergone
unnecessary surgery or invasive therapies as a result of misdiagnosis. The
availability of the ALK1 antibody in 1997 enabled the correct diagnosis of
ALK-positive ALCL, resulting in improvements in targeted therapy and
greater survival rates for patients with this lymphoma. For example, the
five year overall survival rates increased from 71% in 199913
to 89% in 200814. It is expected that additional improvements
in patient survival will continue from future clinical trials. The
widespread introduction of ALK-specific kinase inhibitors is a distinct
possibility.
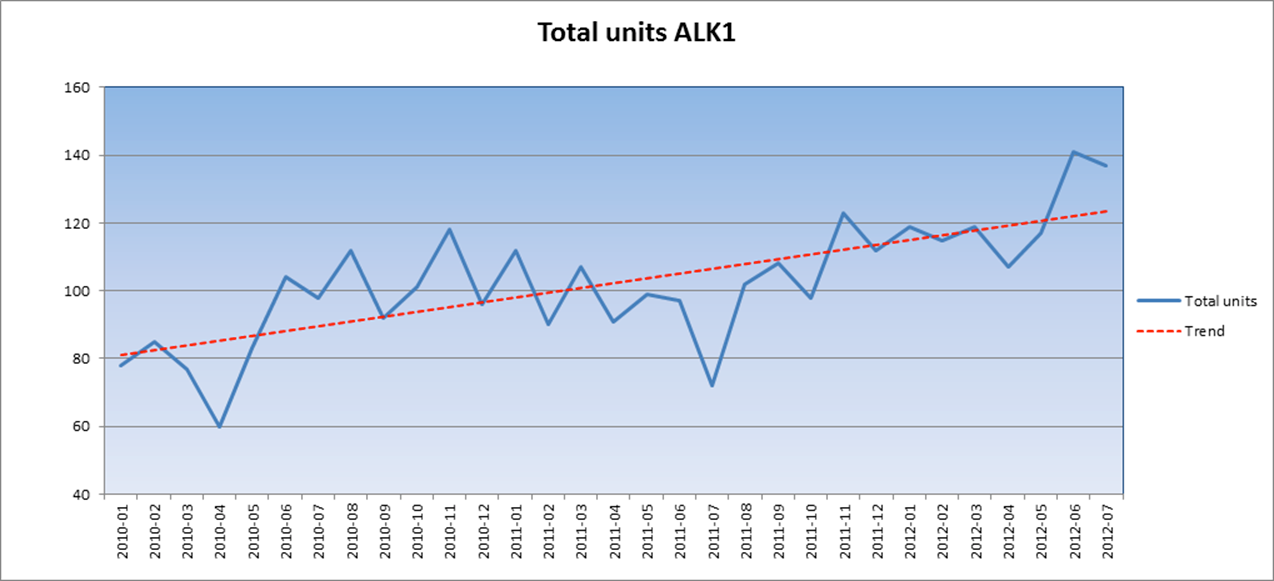
Commercialisation
The ALK1 antibody is licensed commercially throughout the world by
DakoCytomation15 and is considered to be the international gold
standard for identifying this ALK-positive ALCL. The below graph shows the
upward trend in total units of ALK1 sold by Dako from 2010 to 201215.
When patent restrictions are removed in 2014 this upward trend is
predicted to continue.
Total royalties received for ALK reached approximately £50,000 for the
period 1998-2005. Sales of the antibody have since increased due to
worldwide interest in ALK. The total amount of royalties received by the
University of Oxford for ALK from 2008 to 2009 reached £22,875, with
royalties now consistently exceeding £10,000 per annum16.
Sources to corroborate the impact
- Benharroch, D. et al. ALK-positive lymphoma: a single disease
with a broad spectrum of morphology. Blood 91, 2076-84
(1998). First paper in which the antibody ALK1 was used to
identify ALK-positive lymphomas as a distinct entity.
- Delsol, G. et al. Anaplastic large cell lymphoma (ALCL),
ALK-positive. In: Swerdlow, S.H. et al, editors. WHO
Classification of Tumours of Haematopoietic and Lymphoid Tissues.
Lyon. IARC Press; 2008. Reference refers to the description of
ALK-positive ALCL in the current World Health Classification scheme
for Haematological malignancies.
- Kinney, M.C. et al. Anaplastic large cell lymphoma:
twenty-five years of discovery. Arch Pathol Lab Med 135,19-43
(2011). doi: 10.1043/2010-0507-RAR.1. This reference refers an
important update on ALK-positive ALCL.
- NEQAS-ICC. UK Manager. Email statement explaining inclusion of ALK in
the NEQAS lymphoma module labs, received 19th September 2012 (available
on request). Statement confirming use of ALK by NEQAS-ICC.
- Childhood Cancer Statistics. American Childhood Cancer
Organization at
http://www.acco.org/Information/AboutChildhoodCancer/ChildhoodCancerStatistics.aspx
(Accessed 2013) Website for statistics on childhood cancer in the
USA.
- Lymphoma Statistics at http://www.lymphomation.org/statistics.htm#keyfacts
(Accessed 2013) Website for statistics on adult cancer in the
USA.
- Falini, B. et al. ALK+lymphoma: clinico-pathological findings
and outcome. Blood 93, 2697-2706 (1999). Paper
showing increase in 5 year overall survival rates for ALK.
- Lamant, L. et al. Prognostic impact of morphologic and
phenotypic features of childhood ALK-positive anaplastic large-cell
lymphoma: results of the ALCL99 study. J Clin Oncol. 29,
4669-4676 (2011). doi: 10.1200/JCO.2011.36.5411. Reference
describing prognosis in ALK+ALCL where antibody ALK1 was used.
- Monoclonal Mouse Anti-Human CD246, ALK Protein, Clone ALK1. Dako
at
http://www.dako.com/uk/ar38/p118620/prod_products.htm
(Accessed 2013) The antibody ALK1 has been commercialised by
DakoCytomation.
- University of Oxford Finance Division. Royalties Officer. Email
stating royalties for licensed antibodies against ALK, received 30th
July 2012 (available on request). Details of the
commercialisation and upward sales trend of the ALK1 antibody from
DAKO and information on royalties received by the University of
Oxford.