Improving feline health through the worldwide application of infectious and genetic disease polymerase chain reaction assays
Submitting Institution
University of BristolUnit of Assessment
Agriculture, Veterinary and Food ScienceSummary Impact Type
TechnologicalResearch Subject Area(s)
Medical and Health Sciences: Medical Microbiology
Summary of the impact
Bristol University's School of Veterinary Sciences, a global leader in
feline medicine, was the first
UK centre to develop and commercially offer polymerase chain reaction
(PCR) and quantitative (q)
PCR assays to detect a range of feline infectious and genetic diseases.
Since 2008 there has been
a dramatic increase in the number of qPCR tests performed, with over
35,000 tests carried out
between 2008 and 2013. The results of genetic testing have informed
breeding programmes and
resulted in a reduced prevalence of genetic disorders such as polycystic
kidney disease (PKD).
The results of testing for infectious diseases have informed diagnosis and
treatment modalities
and, together with the genetic testing, have contributed to significant
improvements in feline
health and welfare. This work has also generated commercial income
in excess of £1.7M, which
has been used to further research into feline infectious and genetic
diseases.
Underpinning research
The underpinning research was conducted by Dr Chris Helps (Research
Fellow 2001-2007; Senior
Research Fellow 2007-present) and Dr Séverine Tasker, (PhD Student
1999-2002; Lecturer in
Small Animal Medicine 2002-2006; Senior Lecturer in Small Animal Medicine
2006-2013; Reader
in Feline Medicine 2013-present).
Early industry funded studies (2001-2003) by Dr Chris Helps resulted in
the development of feline
herpesvirus [1,4], feline calicivirus [4], Chlamydophila felis
[1,4] and Bordetella bronchiseptica [4]
qPCR assays and their use in samples from over 1700 cats from 218 European
catteries.
Subsequent studies by Dr Séverine Tasker (2001-2002), funded by charitable
trusts, led to the
development of qPCR assays to detect two species of feline haemoplasmas
[2]. More recent
studies (2002-2013) by Drs Chris Helps and Séverine Tasker, funded by
industry and charitable
trusts, led to the development of qPCR assays for the detection of feline
immunodeficiency virus
(FIV) and feline leukaemia virus (FeLV). To date 12 qPCR assays have been
developed for a
range of important feline infectious diseases including the agents causing
cat `flu', conjunctivitis
and anaemia, and 10 PCRs have been developed to detect a range of genetic
diseases such as
kidney [5] and metabolic disorders [6].
Many of the PCR assays described above have been used in national and
international research
projects to study the pathogenesis and determine the prevalence of
infectious organisms in cat
populations. Other research studies using these PCR assays have identified
risk factors for
infection and described the in vivo kinetics of infection with
feline haemoplasmas, monitored
responses to antibiotic treatment [3] and evaluated the potential routes
of transmission. Many of
the PCRs to detect inherited genetic diseases have been used to evaluate
their prevalence in cat
populations [5,6] and to monitor the change in prevalence over time. From
2002-2013, over 30
papers have been published in peer-refereed scientific journals.
Important findings from this research
- PCRs are very sensitive and specific methods for the diagnosis of
feline infectious and
genetic diseases [1,2,4].
- Feline herpesvirus, feline calicivirus, Chlamydophila felis
and Bordetella bronchiseptica are
significantly correlated with upper respiratory tract disease in cats
[4]. Risk factors
associated with these diseases in cats were poor hygiene, contact with
dogs with upper
respiratory tract disease and large numbers of cats housed together.
- From 2005-2013, due to genetic testing and selective breeding, there
has been a 90%
reduction in the prevalence of the mutation causing PKD in the cat
population tested.
- Older male non-pedigree cats are more likely to be infected with
haemoplasmas.
- The feline haemoplasma, Mycoplasma haemofelis, shows cycles of
infection in vivo.
- Marbofloxacin is an effective antibiotic treatment for feline
haemoplasma infection.
- Doxycycline is an effective antibiotic treatment for feline
chlamydophila infection
- Saliva is a possible mechanism of transmission of feline haemoplasmas.
References to the research
[1] Helps, C.R., Reeves N.A., Egan, K., Howard, P. and Harbour, D.A.
(2003) Detection of
Chlamydophila felis and feline herpesvirus by multiplex real-time
PCR. Journal of Clinical
Microbiology. 41:2734-2736. DOI: 10.1128/JCM.41.6.2734-2736.2003
[2] Tasker, S., Helps, C.R., Day, M.J., Gruffydd-Jones, T.J. and Harbour,
D.A. (2003) Use of
real-time PCR to detect and quantify Mycoplasma haemofelis and `Candidatus
Mycoplasma
haemominutum' DNA. Journal of Clinical Microbiology. 41:439-441. DOI:
10.1128/JCM.41.1.439-441.2003
[3] Tasker, S., Helps, C.R., Day, M.J., Harbour, D.A., Gruffydd-Jones,
T.J. and Lappin, M.R.
(2004) Use of a Taqman PCR to Determine the Response of Mycoplasma
haemofelis
Infection to Antibiotic Treatment. Journal of Microbiological Methods.
56:63-71. DOI:
10.1016/j.mimet.2003.09.017
[4] Helps, C.R., Lait, P., Damhuis, A., Björnehammar, U., Bolta, D.,
Brovida, C., Chabanne, L.,
Egberink, H., Ferrand, G., Fontbonne, A., Grazia Pennisi, M.,
Gruffydd-Jones, T., Gunn-Moore, D.,
Hartmann, K., Lutz, H., Malandain, E., Möstl, K., Stengel, C.,
Harbour, D.A. and
Graat, E.A.M. (2005) Factors associated with upper respiratory tract
disease caused by
FHV, FCV, C. felis and B. bronchiseptica in cats —
experience from 218 European catteries.
The Veterinary Record, 156:669-673. DOI: 10.1136/vr.156.21.669
[5] Helps, C.R., Tasker, S., Barr, F.J., Wills, S.J. and Gruffydd-Jones,
T.J. (2007) Detection of
the single nucleotide polymorphism causing feline autosomal-dominant
polycystic kidney
disease in Persians from the UK using a novel real-time PCR assay.
Molecular and Cellular
Probes. 21:31-34. DOI: 10.1016/j.mcp.2006.07.003
[6] Grahn, R.A., Grahn, J.C., Penedo, M.C.T., Helps, C.R. and Lyons, L.A.
(2012) Erythrocyte
Pyruvate Kinase Deficiency Mutation Identified in Multiple Breeds of
Domestic Cats. BMC
Veterinary Research. 8:207. DOI: 10.1186/1746-6148-8-207.
Grants : In addition to a number of small grants from charities,the
following larger grants have
supported research in this area.
[i] 2011-2014 PFIZER ANIMAL HEALTH. £105,000. Tasker, S., Helps, C.R.
& Stokes, C.R.
Feline haemoplasmas: cultivation & defining immunological responses.
[ii] 2007-2010 PFIZER ANIMAL HEALTH. £37,650. Tasker, S. & Helps,
C.R. Evaluation of
haemoplasma pathogenic determinants.
[iii] 2007-2010 UNIVERSITY OF BRISTOL STUDENT PHD SCHOLARSHIP for Barker
E.
£48,600. Tasker, S & Helps, CR. Evaluation of haemoplasma pathogenic
determinants.
[iv] 2006-2010 WELLCOME TRUST. £261,712. Tasker, S. & Helps, C.R.
Pathogenicity, in
vitro culture requirements, transmission and phylogeny of haemotropic
mycoplasmas.
[v] 2001-2003 INTERVET, The Netherlands. £50,000. Harbour, D.A. and
Helps, C.R.
Prevalence of feline pathogens in European catteries detected by real-time
PCR.
Details of the impact
Accurate diagnosis of infectious and genetic diseases is important for
the treatment and
management of cats by vets, as well as to inform breeding policy to reduce
the prevalence of
inherited genetic diseases. The infectious agents for which qPCR assays
have been developed
result in serious and welfare-compromising clinical signs in cats, which
can be fatal if these agents
are not detected and thus not treated appropriately. Upper respiratory
tract disease associated with
feline herpes virus, feline calcivirus, C. felis and B.
bronchiseptica cause ocular and nasal
discharge, pyrexia and anorexia, whilst haemoplasmas cause haemolytic
anaemia. The
retroviruses FIV and FeLV are immunosuppressive agents that are associated
with secondary
infections and neoplasia. The pioneering PCR assays described in the
underpinning research
section have led to significant commercial activity within Langford
Veterinary Services (LVS, a
wholly owned subsidiary of the University of Bristol), in excess of £1.7M
to date, and the following
clinical benefits for affected cats:
An increase in the number of PCR tests
performed leading to specific treatments
The PCR assays are offered to vets and cat breeders
troughout the UK and Europe [a]. By the end of
2013, more than 53,000 PCR tests will have been
performed on over 40,000 cats since testing began in
2002. We have seen a significant increase in the
number of PCR tests run, from ~4000 per annum in
2005-2008 to over 8000 per annum in 2013 [b]
(Figure 1). Accurate detection of feline infectious
agents leads to correct treatment protocols (e.g.
doxycycline for C. felis, antivirals for FHV infection,
marbofloxacin for haemoplasmas) and significantly
improves health outcomes for infected cats. It also allows for appropriate
management (e.g.
isolation of cats, barrier nursing, keeping cats indoors, vaccination of
in-contact cats) to prevent
disease transmission [j].
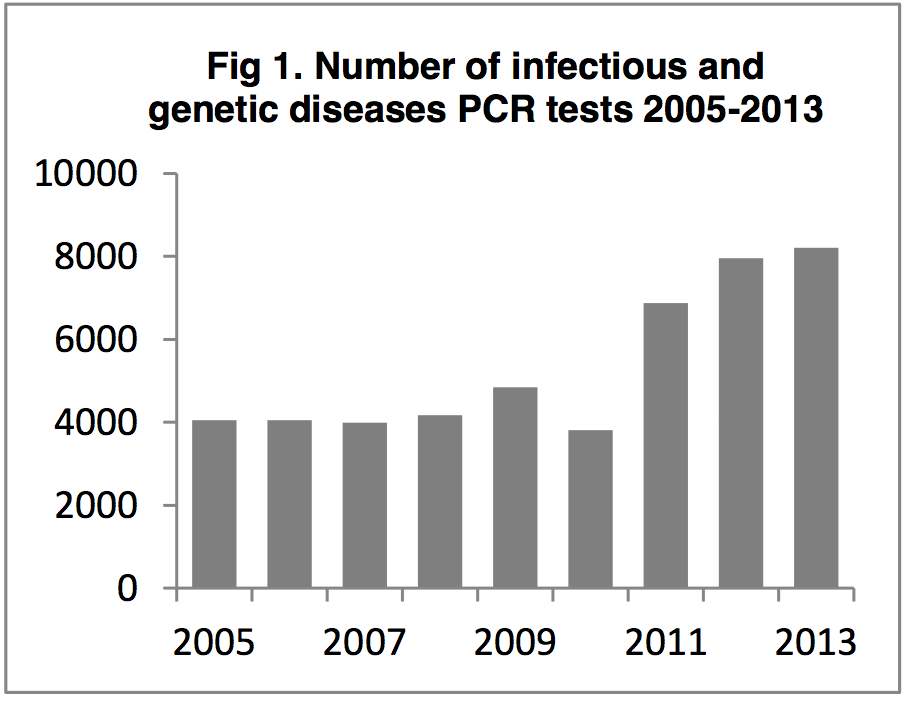
Reduction in the prevalence of inherited genetic
diseases
By genetic testing and selective breeding the
prevalence of inherited genetic disorders can be
reduced. Figure 2 shows the prevalence of the PKD
mutation in 4085 breeding cats, from 735 breeders,
tested between 2005 and 2013 [b]. There has been
a 90% reduction in the number of cats testing
positive for the PKD mutation over the 8 years
Bristol has been running the genetic test, such that
less than 5% of submitted cat samples were PKD
positive in 2013. This indicates that fewer cats will
develop chronic kidney disease due to PKD and
results in a positive impact on feline health and welfare [k].
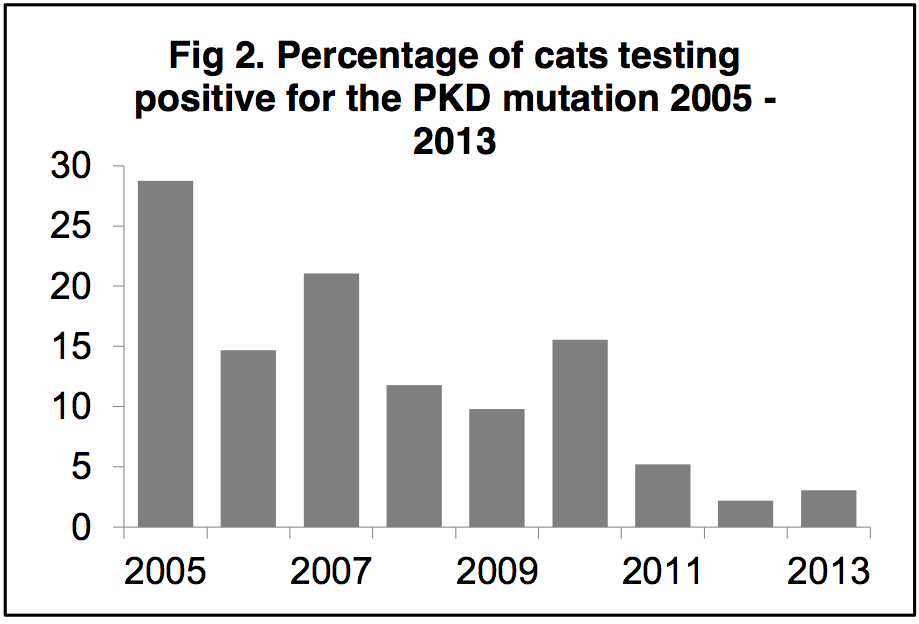
As a result of the Bristol genetic testing service for cat breeders, Dr
Chris Helps has been asked by
the Governing Council of the Cat Fancy (GCCF), the main registration body
for pedigree cats in the
UK, to collaborate on developing breed group genetic disease test panels
[c]. These will form part
of the GCCF Breeder Scheme, which aims to promote responsible cat breeding
and reduce the
prevalence of inherited genetic diseases in pedigree cats.
Number of vets and breeders using the PCR testing service
Since 2009, 705 veterinary practices and 30 referral and University
diagnostic laboratories, both in
the UK and Europe [d], have submitted samples for feline infectious
disease qPCR testing,
showing that a significant proportion (735 out of 4562 (16%, as of 2011))
of UK veterinary practices
use these diagnostic tests. In addition, since 2005 over 1900 [e] cat
breeders across Europe have
submitted samples for genetic disease PCR testing and have used the
results to inform their
breeding policy, with the ultimate aim of reducing or eliminating
inherited genetic diseases in their
breeding lines and increasing feline health. The increase in the number of
infectious disease
samples submitted clearly shows that vets are using these assays, and
hence value the results for
diagnosing and treating cats. Worldwide Guidelines, which include this
Bristol research, are now
available for vets to use to direct therapy and management on the basis of
qPCR results [l,m].
Advice calls and dissemination of information
An audit of advice calls and emails undertaken over a typical week in
November 2012 showed that
32 vets and 10 cat breeders contacted the Diagnostic Laboratory and Feline
Centre regarding
feline infectious and genetic disease PCR testing [f]. This equates to over
2000 advice calls per
year and shows that a significant number of vets and breeders are
aware of and/or use the PCR
assays, value the results they obtain and benefit from talking to
clinicians and geneticists who can
help them interpret the results.
National and international interest in the infectious and genetic PCRs
Figure 3 shows unique page views for feline infectious
and genetic disease PCRs taken from the Langford
Veterinary Services website. Between April 2009 and
November 2012 there were over 26,000 unique page
views on 15 webpages, with the average number of
views increasing from ~3500 in 2009 to ~10,000 in
2012; a 285% increase in 3 years [g]. Looking at the
source of these unique page views shows that 41%
originated outside of the UK, with visits from 126
countries being logged [g]. This level of interest in
feline infectious and genetic disease PCRs shows
both the national and international impact of this work.
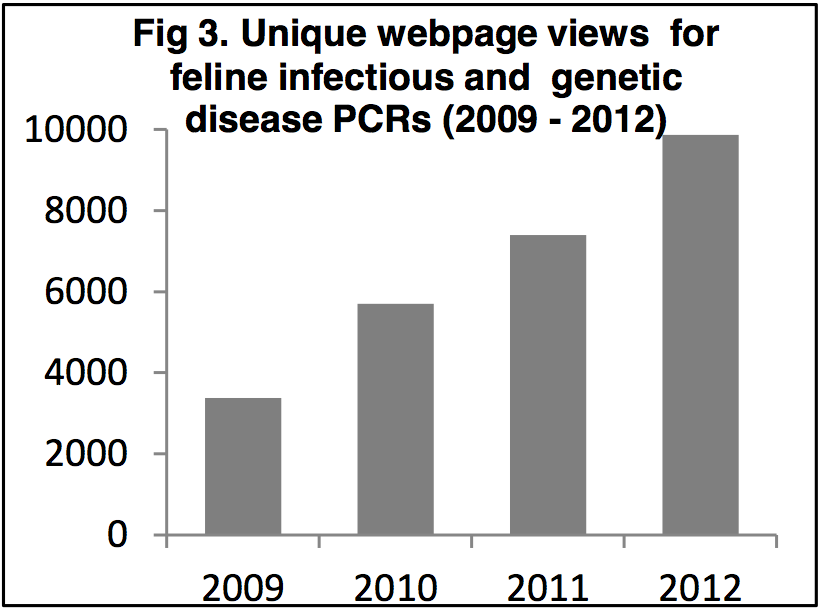
Through sharing reagents derived from the PCR assay development, a number
of other
international laboratories are now offering infectious disease testing by
PCR assays e.g. Iran,
Israel, New Zealand, Portugal, Sweden, Thailand, Turkey, Indonesia,
France, Australia, The West
Indies and USA, enabling cats worldwide to be tested for infectious agents
[h,i].
In conclusion, thanks to Bristol research, qPCR tests for feline
infectious and genetic diseases are
now widely used to benefit feline health and welfare both in the UK and
internationally. Clinical
guidance targeted at veterinary surgeons working in the UK and
internationally now also includes
recommendations to utilise these testing procedures as part of standard
practice [l,m] and the
GCCF is introducing breed-panel genetic testing in collaboration with
Bristol [c].
Sources to corroborate the impact
[a] LVS website, http://www.langfordvets.co.uk/diagnostic-laboratories/diagnostic-laboratories/pcr-acarus
(Corroborates molecular diagnostic testing service available in UK and
Europe)
[b] Excel spread sheets for Fig 1 and Fig 2 data, (data for
Figs1& 2, corroborating large number of
tests run and their change over time and decrease in cats testing
positive for PKD. Full
spreadsheet available via RED, Bristol University)
[c] Letter from the GCCF regarding collaboration and breed specific
genetic testing. (Corroborates
national importance of genetic testing and of Dr Helps' recognised role)
[d] List of veterinary practices, referral laboratories (including
Universities) and cat breeders
sending samples to LVS for disease testing. (Corroborates large number
of users of infectious
disease PCR testing in UK and Europe)
[e] Spreadsheet of cat breeders using the genetic testing service (Corroborates
impact on
behaviour of over 1900 breeders who have used genetic testing to screen
their cats)
[f] Spreadsheet showing advice calls in a typical week (Shows the
importance of qPCR testing and
result interpretation, and its impact on both the vets and breeders who
use the service)
[g] Unique views of the LVS website for the feline infectious and genetic
disease PCR tests (Data
for Fig 3, showing worldwide interest in PCR testing)
[h] Statements from commercial labs, local and international
collaborators. (Responses to
questionnaire describing the international impact of developing tests
with Bristol reagents)
[i] Contact 1, Universiti Putra, Malaysia. (Contact for testimonial
regarding international impact of
PCR test development)
[j] Contact 2, Field Veterinary Officer, Cats Protection, National Cat
Centre (Contact for impact of
infectious disease qPCR testing on cat health, disease treatment
decisions and transmission)
[k] UFAW website. http://www.ufaw.org.uk/POLYCYSTICKIDNEYDISEASEPERSIAN.php
(Describes impact
of genetic testing on disease reduction, quotes Bristol research)
[l] European Advisory Board on Cat Diseases (ABCD) Guidelines published
in Journal of Feline
Medicine and Surgery (2009) 11: Feline herpes virus (547-555), Feline
calicivirus (556-564),
FeLV (565-574) and C. felis (605-609). (Impact via
dissemination of information about PCR
testing as a recommended method)
[m] Canine and Feline Infectious Diseases by Jane E Sykes (2013).
Elsevier, Health Sciences Div.
Ch 5 Nucleic acid detection assays, Ch 22 FeLV, Ch 23 Feline respiratory
viral infections, Ch 33
Chlamydial infections, Ch 41 Hemoplasma infections. SBN 1437707955 (Impact
as for [l])