Accelerating diagnosis of the UK's biggest childhood cancer killer
Submitting Institution
University of NottinghamUnit of Assessment
Clinical MedicineSummary Impact Type
HealthResearch Subject Area(s)
Medical and Health Sciences: Clinical Sciences, Public Health and Health Services
Summary of the impact
The University of Nottingham's Children's Brain Tumour Research Centre
developed new NHS evidence-accredited referral guidelines, published in
2008, to reduce diagnostic delays for children with brain tumours. Their
messages were disseminated through an awareness campaign, `HeadSmart — Be
Brain Tumour Aware', launched in 2011. Three months post-launch, 11% of
the UK population (over 14 million people) and 73% of paediatricians were
aware of HeadSmart, and diagnostic confidence among paediatricians had
risen from 32% to 54%. The time from symptom onset to brain tumour
diagnosis reduced from 14.4 weeks in 2006 to 6.9 weeks in 2013. This
strategy is a `world first' in paediatric brain tumour, now being emulated
internationally.
Underpinning research
Why children's brain cancer? Brain cancers are the commonest solid
tumours for children under 16 years, with around 500 being diagnosed in
the UK annually. Delays in diagnosis are common, risking premature death
in a few and disability in over 60%, mainly due to brain damage occurring
around the time of diagnosis. The risk of disability is greatest for the
youngest, due to their brain's immaturity, and can impair a child's
acquisition of critical skills, thereby impairing their education. Watch:
Jake's Video; Sarah's
Video; All
they need is a scan
Why try to accelerate diagnosis? For patients and families,
reducing unnecessary delays at the critical moment of diagnosis is
important to boost their confidence in health care, and to improve
survival and reduce disability of survivors. As tumours detected earlier
are usually smaller, there is less raised intra-cranial pressure, tumour
invasion and compression of vital structures, and therefore less brain
damage. Symptoms that progress during the pre-diagnostic interval are
consequently less advanced, reducing damage to vision, dexterity,
mobility, cognition and the brain's control of growth and development.
Why did this project happen? The University of Nottingham's
Children's Brain Tumour Research Centre (CBTRC) was established in 1997 to
initiate research focused upon issues prioritised by children with brain
tumours and their families. Public concerns about diagnostic delays,
expressed in the media, in the courts and in Parliament, were very
prominent and resonated with professional concerns. The median total
diagnostic interval (TDI) from symptom onset to diagnosis were reported to
be 12.0-14.4 weeks in the UK, whilst comparable figures in the US and
Poland were 5 weeks, and Canada, Switzerland and Israel were less than 8
weeks.
What research did we do? Professor David Walker, Dr Sophie Wilne,
Ms JoFen Liu, Professor Richard Grundy, Ms Maya Sussman (all University of
Nottingham, 1993-2013), and Professor Colin Kennedy (University of
Southampton), conducted evaluations of regional referral practice in four
English centres (1981-2006) and a regional audit of referral practice. Dr
Thomas Chu and Professor Michel Coleman (both London School of Hygiene and
Tropical Medicine), working with Professor David Walker at the CBTRC,
studied referral practice across the primary : secondary care interface
using linked data sets from the National Cancer Registry, primary (CPRD,
Clinical Practice Research Datalink) and secondary care (HES, Hospital
Episodes Statistics) electronic records from 1997-2007 in under 24 year
olds. Together, these studies showed that the current median TDI in four
English regional units was 14.4 weeks and had not changed over the
previous 25 years [1,2]. We found that multiple ineffective referrals
between home, primary and secondary care were occurring prior to
diagnosis, particularly in patients with the slowest growing tumours. The
symptom groups that progressed most during the interval from symptom onset
to diagnosis were those involving visual and motor functions. We decided
to produce a new clinical referral guideline for the UK, and to develop
and run an awareness campaign.
How did we produce the guideline? We conducted a systematic
literature review and meta-analysis of previous studies describing
symptomatology [3], which, together with information from referral
studies, supported a Delphi consensus process with over 150 experienced
health professionals and parents. 77 consensus statements were accepted,
and supported the AGREE process which was used to draft a new Clinical
Guideline.
What methodology underpinned the awareness campaign?
The awareness campaign was managed according to Quality Improvement (QI)
methodology, and included:
- Rapid resource development using Plan Do Study Act (PDSA) cycles.
- The selection and application of a QI driver target, a median of 5
weeks which equals the best reported TDI data in the USA, for service
change.
- Development of a UK network of Clinical Champions in each of
the children's cancer treatment centres to communicate the aims and
methods of the project with their regional paediatric and primary care
colleagues and collect TDI data from each new case of brain tumour prior
to and after the Campaign launch.
- A developing network of Community Champions, recruited to
disseminate the campaign within local communities and media.
References to the research
2) Wilne S, Collier J, Grundy R and Walker
D et al, 2012. Progression from first symptom to diagnosis in
childhood brain tumours. European Journal of Pediatrics. 171(1), 87-93.
(SC 6) http://dx.doi.org/10.1007%2Fs00431-011-1485-7
Grants
• 09/2003-02/2006 Walker et al `Tracking delays in diagnosis of brain
tumours in childhood'. Samantha Dickson Brain Tumour Trust / Community
Fund (Lottery) £105k
• 09/2006-08/2007 Walker et al `Pathways II Introduction of guidance to
shorten symptom interval' Samantha Dickson Brain Tumour Trust £44,479
• 11/2009 - 10/2011 Walker et al `Brain Pathways: Promoting an earlier
diagnosis of brain tumours in children'. Health Foundation £409k
• 2013-2018 David Walker and Richard Grundy, CBTRC Programme Grant, The
Brain Tumour Charity £1.5m (within this, a programme grant £200k awarded
for HeadSmart post-doctoral fellowship).
Details of the impact
The clinical guideline
The new Clinical Guideline was published by the Royal College of
Paediatrics and Child Health (RCPCH), with endorsements from additional
relevant colleges in 2008. It was awarded NHS Evidence
accreditation in 2010 [a].
The awareness campaign
In line with recommended practice, we planned a dissemination strategy
and acquired funding through a collaborative application involving the
Children's Brain Tumour Research Centre (CBTRC), RCPCH and The Brain
Tumour Charity (formerly the Samantha Dickson Brain Tumour Trust) to the
Closing the Gaps scheme of the Health Foundation. The strategy was
designed to reduce multiple referrals by highlighting symptom clusters in
a handy symptom card for the public and profession, and by signposting to
a decision-support website (www.headsmart.org.uk/home/).
These
use evidence-based criteria for reassurance, timed review
and urgent referral for imaging. The information support website
is linked to relevant NHS websites. Training modules have been developed
within the HeadSmart website as well as an additional module [b] developed
in conjunction with the Royal College of General Practitioners (RCGP),
called `Brain Tumours in Children' (launched from their e-learning
website). There are also Facebook (facebook.com/headsmartcampaign)
and Twitter
(https://twitter.com/HeadSmartUK)
profiles, and a smartphone app (Text SMART to 81400).

The dissemination campaign, consisting of symptom cards, website and
media programme, was launched on 11th June 2011 to the
profession and the public, with political, professional, charity and
Department of Health support. Watch: HeadSmart
Launch video.
Was awareness and confidence raised?
`The awareness of the signs and symptoms of a childhood brain tumours
have dramatically increased since the HeadSmart campaign was launched'
[c]. Around 14 million people were made aware of HeadSmart, equating to
11% of the UK population [d]. Over 650,000 symptom checklist cards have
now been distributed, the website has had over 117,000 visits from
>98,000 unique visitors and is currently (July 2013) experiencing
>12,000 visits from >11,000 unique visitors per month, the Facebook
site has >12,000 likes and Twitter has >1,250 followers [e].
Professional surveys identified that 73% of paediatricians but only 26% of
GPs were aware of HeadSmart after the launch in 2011 [f]. Amongst
paediatricians, confidence in making a brain tumour diagnosis rose from
32% to 54% [f]. In July 2013, the RCGP agreed to disseminate campaign
materials to its 46,000 membership throughout 2013/14 [g].
Were cases diagnosed more quickly?
Through our clinical guideline and HeadSmart campaign, we `effectively
decreased the risk of serious consequences associated with delay through
significantly reducing the time taken to diagnose brain tumours in
children from 13 weeks to 6 weeks' [g].
Comparing total diagnostic interval (TDI) data at 3 time points:
1) In 2006 in 4 centres for one year,
2) In Jan-June 2011 after Guideline publication in 2008
and prior to HeadSmart launch;
3) Post HeadSmart launch until May 2013.
There was a 52% decrease (p=0.001) in median TDI across these three time
points, with a 65% decrease (3 weeks to 1 week) in median time from first
doctor contact to brain scanning. These decreases in median TDI had the
greatest impact in low grade tumours, compared with high grade tumours,
and a trend for centrally placed tumours involving optic pathways compared
with other anatomical sites. The fall in TDI began with the publication of
our clinical guideline in 2008 [g,h], and HeadSmart built on this, `the
time to diagnosis being dramatically reduced from 9.2 to 6.9 weeks in just
two years, summing up the remarkable impact of this campaign' [c].
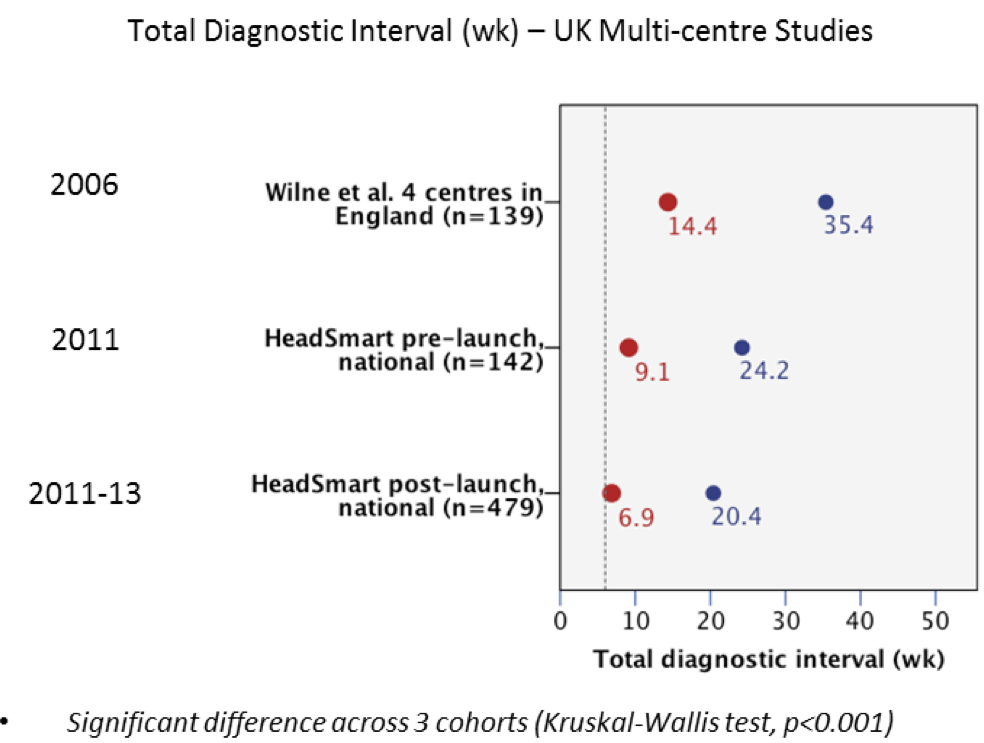 Figure: Time from symptom onset to brain tumour diagnosis has been reduced from a median/mean of 14.4/35.4 weeks (2006) to 6.9/20.4 weeks (2011-2013).
Figure: Time from symptom onset to brain tumour diagnosis has been reduced from a median/mean of 14.4/35.4 weeks (2006) to 6.9/20.4 weeks (2011-2013).
Our preliminary findings on 1 year survival, disability rates, visual
impairment rates, public and professional awareness and website usage, are
the focus of on-going evaluation until 2016, whilst the awareness campaign
is sustained and augmented. As a result of our lobbying, the National
Cancer Intelligence Network announced in 2012 that it will collect TDI
data from children with a brain tumour as part of the cancer registry
dataset from August 2013 [i]. This will benefit researchers and clinicians
nationally, and also facilitate our ongoing evaluation of the impacts of
HeadSmart.
This strategy is a `world first' in paediatric brain tumour, now being
emulated in Germany, Denmark, Sweden, Italy and Iran [g,j]. Its excellence
was acknowledged by an NHS Innovation Award in 2013, worth
£100,000. As summarised by the RCPCH `Overall, this is an excellent piece
of work with wide professional and societal impact and has helped ensure
speedier diagnosis for children with complex conditions as part of
optimising clinical practice' [h].
Sources to corroborate the impact
a. Sophie Wilne, Karin Koller, Jacqueline Collier, Colin Kennedy, Richard
Grundy and David Walker (2010) The diagnosis of brain tumours in children:
a guideline to assist healthcare professionals in the assessment of
children who may have a brain tumour. Archives of Disease in Childhood.
95, 534-539. (SC 18): http://dx.doi.org/10.1136/adc.2009.162057
b. RCGP Training: http://www.elearning.rcg.org.uk/course/info.php?id=99
c. Letter from Neil Dickson, Founder and Vice-Chair, The Brain Tumour
Charity.
d. Media impact report: http://www.cbtrc.org/cbtrc/headsmart/index.aspx
e. HeadSmart dissemination summary. Google analytics: HeadSmart website
traffic data. Provided by Peter Dickens, Head of Communications, The Brain
Tumour Charity.
f. The Health Foundation report (also available as a pdf on
request): http://www.health.org.uk/public/cms/75/76/6270/3930/CtGtCC%20HeadSmart%20final%20report.pdf?realName=UT94qs.pdf
g. Letter from Dr Jane Jones, Assistant Director, The Health Foundation.
h. Letter from Dr Chris Hanvey, Chief Executive, Royal College of
Paediatrics and Child Health.
i. NCIN evidence of NCIN changing strategy to collect TDI data:
http://www.ncin.org.uk/view?rid=1761
(see first 2 bullet points on slide 9)
j. Letter from Kathy Oliver, Co-Director, The International Brain Tumour
Alliance.