Preventing the gastroduodenal hazards of non-steroidal anti-inflammatory drugs and aspirin through widespread adoption of proton pump inhibitors
Submitting Institution
University of NottinghamUnit of Assessment
Clinical MedicineSummary Impact Type
HealthResearch Subject Area(s)
Medical and Health Sciences: Clinical Sciences, Pharmacology and Pharmaceutical Sciences
Summary of the impact
Non-steroidal anti-inflammatory drugs (NSAIDs) are valuable analgesics,
but cause dyspepsia, ulcers and hospitalisation (UK: 3,500pa, USA:
100,000pa) for complications that can lead to death (UK: 400-1,000pa, USA:
16,500pa). Acid inhibition by proton pump inhibitors (PPIs), the only
widely accepted preventative strategy, was proposed and systematically
proved by studies from Nottingham. NICE now recommends PPIs for all
patients using NSAIDs and PPIs are central to all major international
guidelines. PPI co-prescription has increased worldwide (from 27.6% in
2008 to 44.1% in 2012, in the UK); and reduces the risk of hospitalisation
for gastrointestinal bleeding by 54% and symptomatic ulcer by 63%, thereby
preventing up to 540 deaths per annum in the UK.
Underpinning research
Scale of the problem: Non-steroidal anti-inflammatory drugs
(NSAIDs) cause two clinically important gastroenterological problems —
ulcer complications (largely bleeding) which are relatively rare but
dangerous, and dyspepsia which is common, impairs quality of life and
restricts NSAID use. NSAIDs have an attributable rate of hospitalisation
of approximately 2.7-4.0 per 1,000 patient years in patients aged >60.
On the basis of this, prior to widespread adoption of proton pump
inhibitor (PPI) co-prescription, NSAIDs were conservatively calculated to
cause 3,500-4,000 hospitalisations and 400-1,000 deaths pa in the UK
[Pharmacoepidemiol Drug Saf, 2001;10:13-19]. In trials, between 13 and 31%
of patients report dyspepsia [Clin Ther 2010;32:667-677; BMJ
2009;339:b2538].
Nottingham's contribution: Much of the most reliable
epidemiological data that identified and quantified the GI risks of NSAIDs
emanated from the Department of Therapeutics under Professor Michael
Langman (1971-1987). Professor Chris Hawkey (Nottingham Digestive Diseases
Centre, 1983-present) then developed the translational models described
here, becoming a UK leader in this field. Hawkey also developed the
therapeutic interventions discussed, before their evaluation in clinical
trials.
Translational basis: Our translational facility enabled strategies
for mucosal protection and underlying mechanisms to be investigated.
Investigations were based on ex vivo pharmacology, mucosal injury,
spontaneous and induced bleeding and healing of mucosal breaches. Of more
than twenty strategies evaluated, use of omeprazole (first of the then
novel PPI class of drugs) appeared to be most effective. Omeprazole was
potent and reliable in its ability to virtually abolish mucosal injury,
measured as acute microbleeding.
Initial trials: This caused us to suggest to Astra that they
conduct trials with omeprazole in NSAID users, but these suggestions were
not taken up. Our team therefore collaborated with rheumatological and
gastroenterological colleagues in Nottingham and Glasgow to do a proof of
principle investigator-initiated study with the H2 antagonist famotidine.
This study showed that acid inhibition with high, but not standard, doses
of famotidine was effective in preventing and healing ulcers and treating
the dyspepsia caused by NSAIDs1.
Definitive omeprazole trials: This work provoked renewed interest
by Astra. As co-Chief Investigators, Professors Hawkey and Neville Yeomans
(Melbourne, Australia) developed and coordinated a large international
programme of three linked studies of primary and secondary prevention, and
ulcer healing, which the company funded2,3. These studies
showed that omeprazole had clear efficacy and tolerability advantages over
the H2 antagonist ranitidine and the prostaglandin analogue misoprostol,
which were used as comparators. This work authoritatively established the
effectiveness of PPIs for ulcer prevention, healing and maintenance, and
for symptom control, and this was reflected in the ensuing licensed
indications for omeprazole and, later, other PPIs. The nature and quality
of the team's academic relationship led Astra to support a pivotal
investigator-initiated trial of little commercial interest which was
important in showing H.pylori eradication to be insufficient as an
alternative strategy4.
Broadening the evidence: As co-Chief Investigators, Professors
Hawkey and Yeomans later reported similar finding with esomeprazole5,
and effectiveness was shown for other PPIs. Because the GI hazards of
aspirin were an important unmet need, Hawkey and Yeomans persuaded
AstraZeneca to support investigator-initiated studies that established
efficacy of PPI prophylaxis here too6, leading to a successful
regulatory claim for a combination preparation.
References to the research
1. Taha AS, Hudson N, Hawkey CJ, et al. Famotidine
for the prevention of gastric and duodenal ulcers caused by non-steroidal
anti-inflammatory drugs. N Engl J Med 1996; 334(22):1435-1439. http://dx.doi.org/10.1056/NEJM199605303342204
2. Hawkey CJ, Karrasch JA, Szczepanski L, et al.
Omeprazole compared with misoprostol for ulcers associated with
nonsteroidal antiinflammatory drugs. Omeprazole versus Misoprostol for
NSAID-induced Ulcer Management (OMNIUM) Study Group. N Engl J Med 1998;
338(11):727-734
http://dx.doi.org/10.1056/NEJM199803123381105
3. Yeomans ND, Tulassay Z, Juhasz L, Racz I, Howard JM, van Rensburg CJ,
Swannell AJ, Hawkey CJ for the ASTRONAUT Study Group. A
comparison of omeprazole and ranitidine for treating and preventing ulcers
associated with non-steroidal anti-inflammatory drugs. N Eng J Med 1998;
338(11):719-726.
http://dx.doi.org/10.1056/NEJM199803123381104
4. Hawkey CJ, Tulassay Z, Szczepanski L, et al. Randomised
controlled trial of Helicobacter pylori eradication in patients
taking non-steroidal, anti-inflammatory drugs: The HELP NSAIDs Study.
Lancet 1998; 352(9133):1016-1021.
http://dx.doi.org/10.1016/S0140-6736(98)04206-8
5. Hawkey CJ, Talley NJ, Yeomans ND, et al. Improvements
with esomeprazole in upper gastrointestinal symptoms in patients taking
non-steroidal anti-inflammatory drugs including selective COX-2 inhibitors
(NASA1 — SPACE1). Am J Gastroenterol 2005,100(5):1028-1036.
http://dx.doi.org/10.1111/j.1572-0241.2005.41465.x
6. Yeomans N, Lanas A, Labenz J, van Zanten SV, van Rensburg C, Racz I,
Tchernev K, Karamanolis D, Roda E, Hawkey C, Naucler E,
Svedberg LE. Efficacy
of esomeprazole (20 mg once daily) for reducing the risk of
gastroduodenal ulcers associated with continuous use of low-dose
aspirin. Am J Gastroenterol. 2008,103(10):2465-2473. http://dx.doi.org/10.1111/j.1572-0241.2008.01995.x
Relevant grants total over £2m, from Astra, Astra Zeneca, Merck
Sharpe & Dohme, MRC ROPA, Novartis and University of Dundee/EMEA (via
unrestricted grant from Pfizer). All awarded to CJ Hawkey for work between
1993 and 2013 for research on NSAID complications and their prevention,
including co-prescription of NSAIDs and PPIs.
Details of the impact
Our research has had an impact in six main areas: patient safety, quality
of life, healthcare costs, management guidelines, prescribing practice and
opportunities for the pharmaceutical industry.
Patient safety:
Before our research, the only way to reduce the risk of a non-steroidal
anti-inflammatory drug (NSAID)-associated ulcer complication was to avoid
using NSAIDs or to use low doses. Previously recommended measures such as
the use of slow release or effervescent preparations or enteric coating
were at best ineffective. NSAIDs were regarded as the biggest iatrogenic
cause of hospitalisation and death worldwide. The most conservative
estimates (UK) were that 1 in every 250-370 people being treated with
NSAIDs would be hospitalised for ulcer complications each year
[Pharmacoepidemiol Drug Saf, 2001;10:13-19]. With at least a 10% death
rate, this resulted in an estimated societal burden of 3,500-4,000
admissions and 400-1,000 deaths per annum. Estimates from other societies
and meta-analyses of clinical trials suggested greater harm, with annual
ulcer complication rates of 1 in 67 users, with two estimates of death
rates in the USA of 7,000-10,000 [Clin Ther 2010;32:667-677] and 16,500
[Gastroenterol, 1985;96,647-655].
Based on a Health Technology Appraisal (HTA) meta-analysis drawing on our
work [HTA 2006, 10(38); Am J Gastroenterol 2006,101:701-710] and studies
by others with other proton pump inhibitors (PPIs), the NICE
Osteoarthritis Development Group reported in 2009 that use of PPIs in
patients aged 55 or over reduced the risk of hospitalisation for
gastrointestinal bleeding by 54% and symptomatic ulcer by 63%, and was the
most cost effective prophylactic strategya,b. A pro rata
reduction in death could prevent between 216 and 540 deaths per annum in
the UK, with higher values if estimates from other countries are used.
Worldwide, NSAID use is extensive [PLoS Med 2013;10(2):e1001388]. Using
even the most conservative estimate above (1 hospitalisation per 370 users
per annum), several hundred thousand life-threatening ulcer complications
could be prevented annually, worldwide, by use of proton pump inhibitors.
Quality of Life:
As well as preventing ulcer complications, PPIs improve quality of life
by reducing dyspepsia and allowing continuation of treatment that would
otherwise be stopped. NICE estimate dyspepsia rates between 5.4% and 9.6%
per annum in NSAID users and a 57% reduction with PPI co-prescriptiona,b.
Another meta analysis reported higher rates (13.5%-31%) with the 66%
reduction by PPIs being identified as the most effective available
treatment or preventative measure for NSAID dyspepsia [Am J Med;
2006:119(5):448.e27-36]. NICE estimate that the combined effect of reduced
mortality and improved quality of life results in a gain of 5-10 QALYs
(quality adjusted life years) per thousand people treateda,b.
[QALYs are a measure of disease burden used to assess the value of a
medical intervention. They are based on the number and quality of years of
life that would be added by the intervention. One QALY is one year spent
in perfect health.]
Healthcare costs:
NICE report that the ability of PPIs to prevent hospitalisation and other
adverse events reduces healthcare costs to the extent that their use
`increases the estimated gain in quality adjusted life years at little or
no additional cost" with "savings from not having to treat adverse
effects'.a
Guidelines:
These observations led NICE to recommend that PPIs are considered for use
in all patients taking NSAIDsa. All other major guidelines
produced or updated in the past five years (Osteoarthritis Research
Society International [OARSI] 2008c, Cardiology 2008d,
American Colleges of Gastroenterology 2009e, and Rheumatology
2012f, place the PPI co-prescription strategy that we developed
at the heart of their guidelines, particularly for patients at increased
risk of ulcer complications. An updated Cochrane analysisg
supports the strategy, as do other national and international
recommendations.
Prescribing practice:
To be effective, guidelines must be implemented.
Current data show progressive adoption of PPI co-prescription in UK (see
figure) and internationally [Am J Gastroenterol. 2008;103:1097-1103].
Clinical Practice Research Database Statisticsh show a rise in
co-prescription of a PPI in the UK from 27.6% in 2008 to 44.1% in 2012 in
patients aged >45 using non-aspirin NSAIDs, resulting in safer symptom
relief. During this time, aspirin use has doubled in the UK with a
concurrent rise in the proportion of patients receiving PPI protectionh
from 25.5% in 2008 to 34.8% in 2012, allowing patients to access the
cardiovascular and anti-cancer benefits of aspirin more safely.
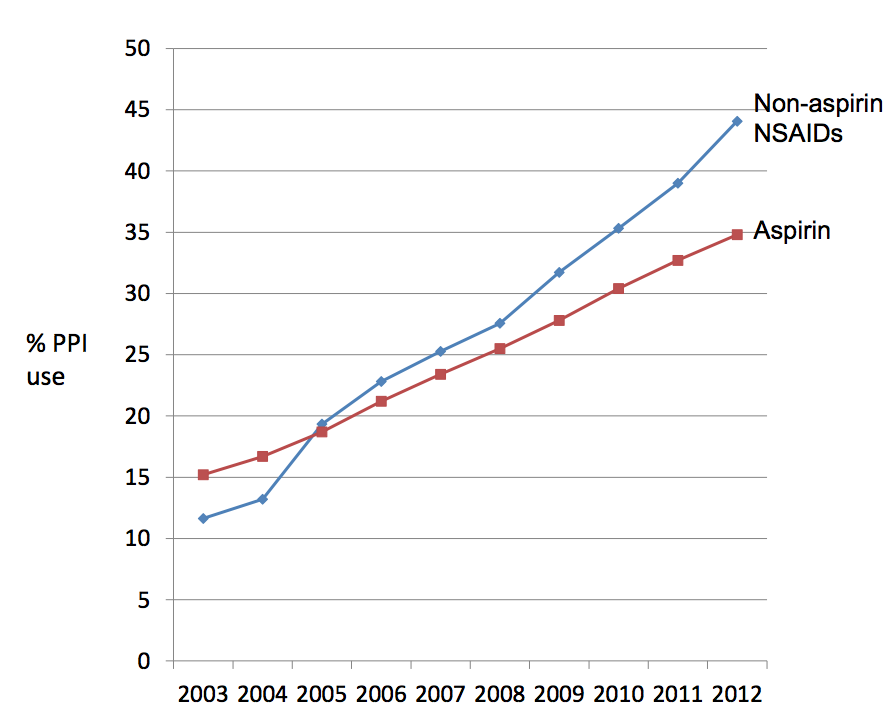 Figure: Rates of UK co-prescription of PPIs in patients using NSAIDs and aspirin by year, based on data routinely collected from general practices with electronic systems that feed into the Clinical Practice Research Database.
Figure: Rates of UK co-prescription of PPIs in patients using NSAIDs and aspirin by year, based on data routinely collected from general practices with electronic systems that feed into the Clinical Practice Research Database.
Commercial Opportunities:
Esomeprazole is one of AstraZeneca's 10 leading medicines by sales.
Worldwide sales of this drug generated £3.9 billioni in revenue
for AstraZeneca in 2012, with an increasingly significant contribution
from NSAID ulcer prophylaxis. The effectiveness of PPI prescription has
also led to development of combination preparations, of which several have
so far been approved and launched internationally (Axorid: ketoprofen +
omeprazole 2009, Vimovo: naproxen + esomeprazole 2010, and Axanum: Aspirin
+ esomeprazole:2011)j.
Sources to corroborate the impact
a) Latimer N. Lord J. Grant RL. et al. National Institute for Health and
Clinical Excellence Osteoarthritis Guideline Development Group. Cost
effectiveness of COX 2 selective inhibitors and traditional NSAIDs alone
or in combination with a proton pump inhibitor for people with
osteoarthritis. BMJ. 339:b2538, 2009 http://dx.doi.org/10.1136/bmj.b2538
b) National Institute for Health and Clinical Excellence. Osteoarthritis:
The care and management of osteoarthritis in adults. http://www.nice.org.uk/nicemedia/pdf/CG59NICEguideline.pdf
c) Zhang W, Moskowitz RW, Nuki G, et al. OARSI Recommendations for the
Management of Hip and Knee Osteoarthritis, Part II: OARSI evidence-based,
expert consensus guidelines. Osteoarthritis & Cartilage 2008; 16:
137-162.
http://dx.doi.org/10.1016%2Fj.joca.2007.12.013
d) American College of Cardiology Expert Consensus Document on Reducing
the GI risks of Antiplatelet Therapy and NSAID Use 2008:
http://cardiocompass.cardiosource.org/cc2/openCont2?objID=108
e) Lanza FL, Chan FKL, Quigley EMM and the Practice Parameters Committee
of the American College of Gastroenterology. Guidelines for prevention of
NSAID-related Ulcer Complications. Am J Gastroenterol 2009; 104: 728-738.
http://dx.doi.org/10.1038%2Fajg.2009.115
f) Hochberg MC, Altman RD, April T, et al, American College of
Rheumatology 2012 Recommendations for the use of Nonpharmacologic and
Pharmacologic Therapies in Osteoarthritis of the Hand, Hip and Knee.
Arthritis Care & Research 2012; 64(4): 465-474.
http://dx.doi.org/10.1002%2Facr.21596
g) Rostom A. Dube C. Wells G. et al. Prevention
of NSAID-induced gastroduodenal ulcers. Cochrane Database of
Systematic Reviews http://dx.doi.org/10.1002/14651858.CD002296
h) Scrutiny of CPRD database. Corroborated by Dr Yana Vinogradova (pdf
available on request).
i) AstraZeneca 2012 annual report: http://www.astrazeneca-
annualreports.com/2012/documents/eng_download_centre/annual_report.pdf
j) Vimovo: http://www.medicines.ie/medicine/14981/SPC/VIMOVO+500+mg+20+mg+modified-release+tablets/#ORIGINAL