Development of long-acting antimicrobial implantable devices that prevent disabling infections, cut healthcare costs and reduce bacterial resistance
Submitting Institution
University of NottinghamUnit of Assessment
Clinical MedicineSummary Impact Type
TechnologicalResearch Subject Area(s)
Biological Sciences: Microbiology
Engineering: Biomedical Engineering
Medical and Health Sciences: Medical Microbiology
Summary of the impact
The use of implantable polymeric devices is limited by infection.
University of Nottingham research led to patented technology for
hydrocephalus shunts that provides biomaterials with long-acting
antimicrobial action. Almost 70% of shunts used annually in England now
comprise our [text removed for publication] shunt, and UK usage has grown
by 22% since 2008. The technology has reduced infection rates from 8.75%
(2008) to 3.6% (2013), and prevents around 370 brain infections and 38
deaths in England each year. This is saving NHS England an estimated
£18.4m in treatment costs each year, and generating company revenue.
Furthermore, our [text removed for publication] EVD catheters for
temporary relief of intracranial hypertension have reduced the rate of
brain infections from 7.6% to 0.9%.
Underpinning research
Biomaterials-associated infection is a common complication of surgical
implant devices, regardless of the biomaterial used. These infections are
highly resistant to the immune system and to antimicrobials. In
hydrocephalus, where treatment is by insertion of a shunt or temporary
external ventricular drain (EVD), infections account for one-third of all
shunt-related mortality and worsen the overall prognosis. Despite
modifications in surgical technique, the incidence of cerebrospinal fluid
shunt infection remains unacceptably high at 5-15%, and in EVD this can
reach 25-30% of cases. Even when successfully treated, infections are
associated with reduced intelligence and cognition, increased risk of
seizures and psychomotor retardation.
In 1993, Professor Roger Bayston began a research programme in the
Biomaterials-Related Infection Group at the University of Nottingham to
address this problem. In researching appropriate antimicrobial
biomaterials for shunts, we discovered that individual infecting bacteria
attached to the biomaterial and developed highly adherent and resistant
biofilms. We showed that eradication of biofilm bacteria requires
prolonged exposure to local high antimicrobial concentrations and that
this needs to be sited at the interface of bacteria and implant polymer
[1]. Since antimicrobial coatings do not give sufficient duration of
activity, we developed a novel process [text removed for publication] that
allowed devices to be impregnated, not just coated, with antimicrobials
post- manufacture. In this way, the antimicrobials remain evenly
distributed in the polymer matrix as molecules rather than drug particles,
giving more controlled release and improved mechanical properties.
Crucially, we designed the process to enable the molecules to migrate
freely through the crosslinked polymer, thus repeatedly replenishing the
surface layer. The manufacturing process permitted antimicrobial devices
to withstand sterilisation by autoclaving or ethylene oxide. The patented
technology has been commercialised only by the University of Nottingham
and is unique [2]. Studies of the mode of action of the antimicrobial
biomaterial showed that bacteria attached to the polymer and were then
killed. But approximately 48hrs were needed to kill all the bacteria and
antimicrobial coatings are rapidly depleted by fluid flow before this
time-point, hence explaining the failure of these coatings. The
bactericidal activity of the [text removed for publication] shunt was
found to last for 50 days using rifampicin and clindamycin, compared with
three days for existing technologies. This was sufficient to avoid
infection in the crucial first month after shunt implant in hydrocephalus
patients. Safety studies were carried out [3] and commercialisation of the
shunt by [text removed for publication] has led to clinical evaluation and
use (see Section 4).
The process was further adapted to cover EVD and peritoneal dialysis
catheters with a long period of infection risk and with a need for broader
spectrum antimicrobial activity (4,5). In both cases, where biofilm growth
occurs both in the lumen and down the outside, 80-100 days effectiveness
against a range of pathogens, including Staphylococcus epidermidis,
MRSA, anaerobes and multi-drug-resistant Gram negative bacteria such as
ESBL E coli and Acinetobacter, was achieved. These modifications
were patent protected [2]. Our recently developed long-term urinary
catheter (Fisher, Ashraf and Bayston, in preparation) is also active for
three months against these pathogens, as well as against Proteus
mirabilis, known as the scourge of catheterised spinal injuries
patients.
References to the research
1. Bayston R, Ashraf W, Bhundia C. Mode of
action of an antimicrobial biomaterial for use in hydrocephalus shunts.
2004 J Antimicrob Chemother; 53: 778-782.
http://dx.doi.org/10.1093/jac/dkh183
2. Bayston R. 2007. Medical devices
and methods of making medical devices. EP1804845 (W02006032904) Europe.
3. Abed WT, Alavijeh MS, Bayston R,
Shorvon S, Patsalos PN. An evaluation of the epileptogenic properties of a
rifampicin / clindamycin — impregnated shunt catheter. Br J Neurosurg
1994; 8: 725-730.
http://dx.doi.org/10.3109/02688699409101187
5. Bayston R, Vera L, Ashraf W.
Activity of an Antimicrobial Hydrocephalus Shunt Catheter against
Propionibacterium acnes. Antimicrob Ag Chemother 2010; 54: 5082-5085.
http://dx.doi.org/10.1128/AAC.00540-10
Patents filed on this technology since 1993 — Medical devices and methods
of making medical devices (Bayston, R):
| UK |
0303033.5 & 0421164.5 |
|
|
| PCT |
GB2005/003667 |
JP |
2007-532961 |
| Euro |
05781960.9 |
CA |
2 580 894 |
| US |
11/690 567 |
MYA |
PI.20070453 |
| Euro Div1 |
10178080.7 |
Euro Div2 |
10178073.2 |
Details of the impact
Clinical and patient benefits
[text removed for publication] shunts and External Ventricular Drain
(EVD)
The initial application of our novel approach to anti-infective catheters
(USA Patent 4917686) was licensed in 1995 to a major USA company [text
removed for publication]. Since launch (1998 in EU, 2001 in USA), about
97,000 children and adults in 47 countries have received the antimicrobial
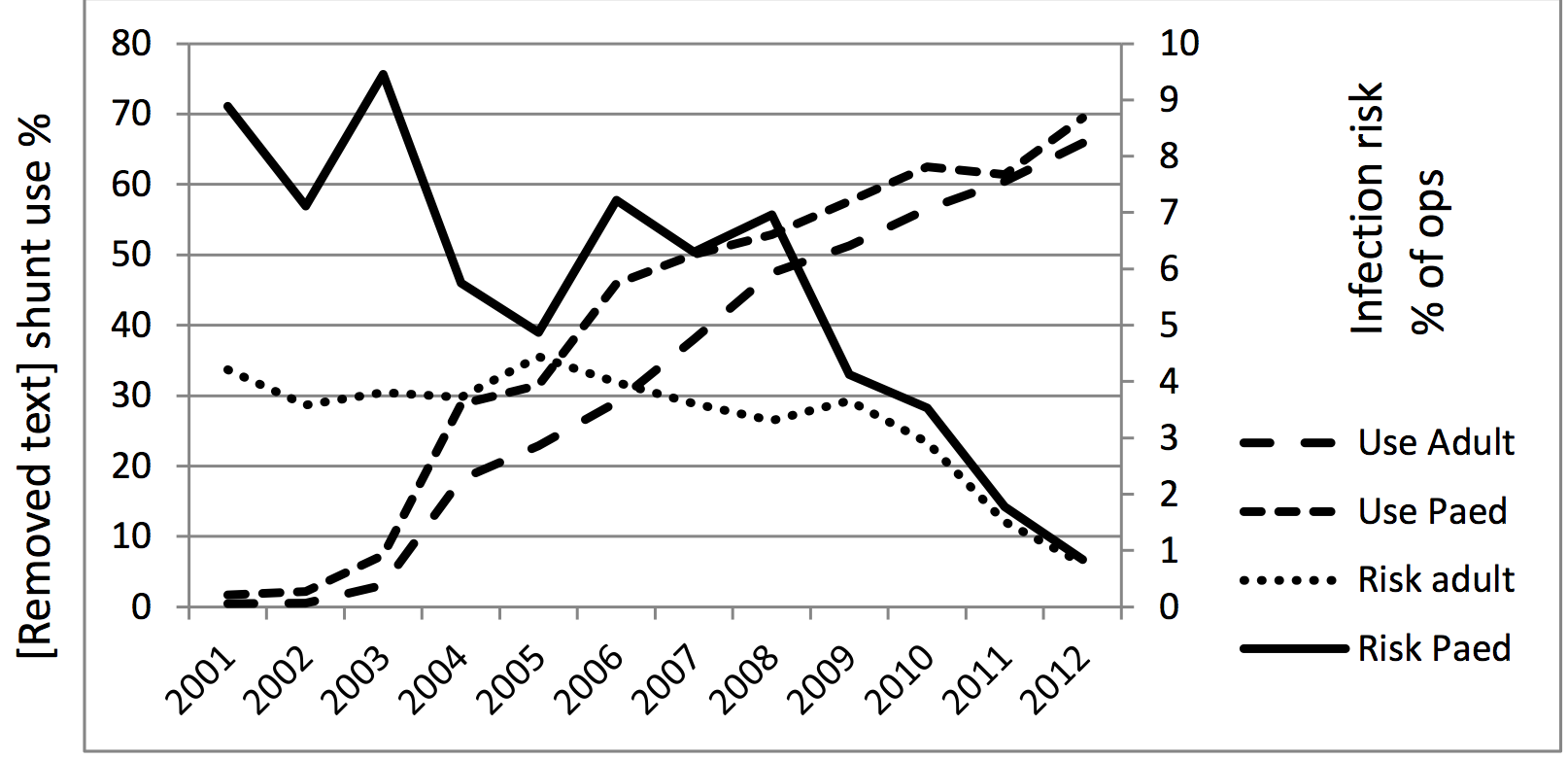
shunt, and since 2008 there has been a 22% growth in usage in the UK [a].
Almost 70% of shunts used annually in England now comprise our [text
removed for publication] shunt [a] and the technology prevents around 370
brain infections and 38 deaths in England each year [b]. Eleven
international clinical trials involving 5,613 patients [c] (2,180 since
2008) have all shown a reduction in infection (control rate 8.75%, [text
removed for publication] rate 3.6%, extracted from those studies after
2008), and data from the UK Shunt Registry [J Neurosurg Pediatr 2009;
4(4): 389-393] has confirmed this. In the same analysis, infection rates
for paediatric shunts were: control 5.8%, [text removed for publication]
0.9% [c]. [text removed for publication] is currently the subject of
application for regulatory approval by the Chinese FDA, in which Bayston
plays a pivotal role, having face-to-face meetings with Chinese officials.
In addition, the observed patient benefits of [text removed for
publication] have influenced the Department of Health's National Institute
for Health Research (NIHR), causing them to invest in a large multicentre
clinical trial of [text removed for publication] shunts (the BASICS trial;
£2.04M http://www.nets.nihr.ac.uk/projects/hta/1010430).Bayston
has declined to be involved in this trial in order to avoid bias, but has
given advice on diagnostic criteria and microbiological investigations.
In 2001, the technology was licensed for catheters for external ventricular
drainage (EVD), a temporary means of 3 Use Adult intracranial Use Paed
pressure control. Since launch in Risk adult 2002 390,000 Risk Paed patients
have benefitted from the new EVD. In 2010, we licensed our technology
extending the antibacterial spectrum for EVD to cover multi-drug-resistant
Gram negative bacteria such as ESBL E coli and Acinetobacter to
[text removed for publication]. A 2010 study has shown a significant
reduction in brain infection, from 7.6% to 0.9%, using [text removed for
publication] EVD [d]. Use of our EVD catheter applies prophylactic
antibiotics only at the site of bacterial exposure, and a 2010 study has
shown that [text removed for publication] significantly reduces the need for
systemic antibiotics, so reducing their adverse events including Clostridium
difficile infection (an increasing antibiotic-related problem
worldwide resulting in superinfection and often need for colectomy) [J
Neurol Neurosurg Psychiatry 2010,81:1064-7]. [text removed for publication]
therefore has an important role in cutting antibiotic use and reduction of
associated risk.
The current beneficiaries of the [text removed for publication] research
are primarily neurosurgical patients with hydrocephalus or head trauma,
who require intracranial pressure management, and who have been shown to
experience significantly fewer episodes of ventriculitis, abdominal sepsis
and other complications. In the US, approximately 33,000 patients are
shunted each year costing $100 million [Ped Neurosurg 1995,23: 254-259].
Clinical trials of [text removed for publication] shunts since 2008 have
shown a reduction in infection rate from 8.75% to 3.6% [c]. Treatment of
shunt infections is by shunt removal and systemic antibiotics, and, after
2-3 weeks, insertion of a new shunt. In up to 26% of cases this needs to
be repeated due to infection recurrence [J Neurosurg (Ped)
2006;105,177-181]. Therefore, applying the reduction in infection rate of
8.75% to 3.6% [c] to the 198,000 shunts inserted in USA since 2008, just
over 10,000 patients would have been spared at least two, and possibly
four or more, extra operations had [text removed for publication] been
used throughout. Similar results (reduction in infection rate 7.6% to
0.9%) are seen when our antimicrobial EVD catheter is used in
neurocritical care [d].
Dialysis and urinary catheters
Our modifications to the technology for other applications requiring
broad antimicrobial spectrum and long duration have led to other licensing
deals. The rights to our catheter for reducing peritonitis in Continuous
Ambulatory Peritoneal Dialysis (CAPD) patients, with activity against
staphylococci and Gram negative bacilli, were licensed to USA company
[text removed for publication] in 2005, and this catheter is now
undergoing CE Marking. Since 2009, the company has invested approximately
$1.337M in new construction and production equipment and employed one full
time engineer and two part-time regulatory / marketing executives
specifically for this venture. The company have decided to name the
product the `Bayston Catheter' [e,f].
Beneficiaries of the CAPD catheter are those with end-stage renal disease
(ESRD) who are expected to experience significantly fewer episodes of
peritonitis and fewer catheter changes (each a surgical procedure).
Approximately 1m people in the USA and probably several times this figure
in Korea and China are affected. Worldwide, an average of 11% of ESRD
patients are treated with CAPD, but in EU and Korea, CAPD is used more
often, and 75% of ESRD patients in Mexico use CAPD [J Am Soc Nephrol
2012;23,533-544].
Long-term urinary catheters are used in tens of millions of patients
worldwide, with infection rates around 40%. We are working with the
international devices company [text removed for publication] to evaluate
our recently developed urinary catheter for regulatory approval.
Training senior surgeons
The research surrounding the base technology and its clinical
applications has underpinned Bayston's contributions to the DePuy
Hydrocephalus and Neurocritical Care Learning Centre (http://www.depuy.com/uk/healthcare-professionals/education-and-training),
held three times each year for senior professionals in Hamburg, Istanbul,
Neuchatel and Prague. Since 2008, approximately 400 senior surgeons have
attended, and Continuing Professional Development (CPD) points are awarded
for these sessions at rates depending on national systems. As an example,
a 3-day training session in Neuchatel in 2010 was attended by 61
professionals from South America, EU, Eastern Europe, Africa, Russia and
Korea. Up-to-date best practice is taught and discussed so that delegates
return to their home countries in a position to institute regimens to
reduce complications and improve patient outcome.
Commercial benefits
Cost-savings for the NHS
One large USA [text removed for publication] shunt study [g] showed a
reduction in infection rate from 12% to 3.2%, a reduction of 53 days'
hospital stay for every 100 patients shunted, and an associated saving of
$442,133 per 100 patients shunted. In one German hospital, use of the
antimicrobial shunt catheters led to reduction in infection rate from 5.8%
to 1%, yielding annual savings of $1.3m [h]. In England, the technology
prevents around 370 brain infections and 38 deaths each year, thereby
saving NHS England an estimated £18.4m in treatment costs annually [b].
Costs of infections vary between countries and institutions, but an
estimated $100m annual cost worldwide of shunt complications (which
includes non-infective causes) would be reduced by $35m-$50m by the use of
[text removed for publication] shunts (calculated from figures in the
literature).
Sources to corroborate the impact
a. Cambridge shunt registry data. Supplied in confidence by [text removed
for publication]. Available on request.
b. Economic analysis by Professor R Elliott, Lord Trent Professor of
Medicines and Health. The University of Nottingham.
c. Parker SL, Anderson WN, Lilienfeld S, Megerian JT, McGirt MJ.
Cerebrospinal shunt infection in patients receiving antibiotic-impregnated
versus standard shunts. J Neurosurg 2011; 8: 259-265. http://dx.doi.org/10.3171/2011.6.PEDS11257
d. Harrop JS, Sharan A, Ratliff J, et al. Impact of standardized protocol
and antibiotic-impregnated catheters on ventriculostomy infection rates in
cerebrosvascular patients. Neurosurg 2010; 67: 187-191. http://dx.doi.org/10.1227/01.NEU.0000370247.11479.B6
(pdf available on request).
e. Email correspondence from [text removed for publication]; and Gary
Evans, Head of IP Management and Legal Services, BEIS, The University of
Nottingham.
f. Letter from [text removed for publication], President, [text removed
for publication].
g. Attenello FJ, Garces-Ambrossi GL, Zaidi HA, Sciubba DM, Jallo GI.
Hospital costs associated with shunt infections in patients receiving
antibiotic-impregnated shunt catheters versus standard shunt catheters.
Neurosurg 2010; 66: 284-289 (pdf available on request). http://dx.doi.org/10.1227/01.NEU.0000363405.12584.4D
h. Eymann R, Chehab S, Strowitzki M, Steudel WI, Kiefer M. Clinical and
economic consequences of antibiotic impregnated cerebrospinal fluid shunt
catheters. J Neurosurg Pediatr 2008; 1: 444-450. http://dx.doi.org/10.3171/PED/2008/1/6/444