Parkinson’s Disease – recognition, quantification and treatment of non-motor features
Submitting Institution
University College LondonUnit of Assessment
Psychology, Psychiatry and NeuroscienceSummary Impact Type
HealthResearch Subject Area(s)
Medical and Health Sciences: Clinical Sciences, Neurosciences
Summary of the impact
We established a comprehensive international collaboration to develop,
validate and apply new scales for the identification and quantification of
non-motor symptoms and signs in Parkinson's disease (PD). This was
intended to provide tools to assess response to treatment, help define the
clinical prodrome of PD and provide a means to evaluate novel therapies
designed to modify the course of disease. The scales have been produced,
validated and published. They have been incorporated as end points in
international clinical trials for PD and have been introduced by
specialist societies and NHS commissioners as a standard of care for PD
patients.
Underpinning research
UCL has developed a major research programme in Parkinson's disease. This
encompasses the basic sciences, clinical research and clinical trial
development and execution. Parkinson's disease is a neurodegenerative
condition that affects multiple areas of the brain as it progresses and
produces motor symptoms (bradykinesia, rigidity, tremor) and a range of
non-motor features that include cognitive impairment, autonomic
dysfunction, psychiatric disturbances etc. [1]. These non-motor
features dominate the patient's quality-of-life in mid to advanced
Parkinson's disease. In addition, it has been recognised that certain
non-motor features may precede the onset of motor symptoms and therefore
the diagnosis of Parkinson's disease. This emphasised the potential
importance of non-motor features in the pre-motor clinical prodrome of
Parkinson's disease [2].
The Department of Clinical Neurosciences has played a major role in
research into the cause and treatment of Parkinson's disease and was the
first to describe the mitochondrial contribution to this and other
neurodegenerative diseases. From 1993, the Department led research on
defining the role of mitochondria in neurodegeneration, specifically in
Parkinson's disease, Huntington's disease and in Friedreich ataxia,
publishing several seminal articles which are now citation classics
(citations >400). As part of this research the pattern of
neurodegeneration in these diseases and the corresponding clinical
deficits became more clearly defined, and the Department played a leading
role in this particularly in Friedreich ataxia. Novel scales for the
assessment of ataxia and the impact on patient quality of life were
developed. As part of this exercise, it became clear that there was also
an unmet need in this area, specifically in the context of Parkinson's
disease and its non-motor symptoms.
In 2006, the Department organised and co-chaired with Dr K Ray Chaudhuri
(King's College London) the creation and development of the International
Non-Motor Study Group for Parkinson's disease. The intention of this group
was to develop patient-reported symptom scales and physician-recorded
non-motor sign scales for the assessment and quantitation of non-motor
features for use in clinical management, research and clinical trials.
The study group produced the non-motor symptom questionnaire (NMSQuest)
and the non-motor symptom scale (NMSS) [3]. These were rigorously
assessed for validity and reproducibility, and validated in several
European and Asian languages. Both the questionnaire and symptom scale
have been used in clinical trials as endpoints in the assessment of novel
therapies for Parkinson's disease.
The questionnaire and scale have also been assessed in research to define
the clinical prodrome of Parkinson's disease, particularly in certain
stratified groups such as those with glucocerebrosidase mutations. This
research has demonstrated that selective cognitive impairment and hyposmia
precede the onset of motor dysfunction in this genetically determined
group. The non-motor questionnaire and symptom scale are now being used in
conjunction with imaging to select those amongst this group who are
particularly at risk of developing Parkinson's disease. These individuals
will then be offered participation in a clinical trial of novel small
molecule chaperones to enhance glucocerebrosidase activity and reduce
alpha-synuclein levels [4]. Research from UCL suggests that such
therapy may be generally applicable across the Parkinson's disease
aetiology spectrum.
Thus the development of the non-motor symptoms scales has had significant
implications for patient management, an understanding of the evolution of
Parkinson's disease, clinical trials and in particular the development of
disease modifying therapies.
References to the research
[1] Chaudhuri KR, Martinez-Martin P, Brown RG, Sethi K, Stocchi F, Odin
P, Ondo W, Abe K, Macphee G, Macmahon D, Barone P, Rabey M, Forbes A,
Breen K, Tluk S, Naidu Y, Olanow W, Williams AJ, Thomas S, Rye D, Tsuboi
Y, Hand A, Schapira AH. The metric properties of a novel non-motor
symptoms scale for Parkinson's disease: Results from an international
pilot study. Mov Disord. 2007 Oct 15;22(13):1901-11. http://dx.doi.org/
10.1002/mds.21596
[2] Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, MacPhee
G, Brown RG, Naidu Y, Clayton L, Abe K, Tsuboi Y, MacMahon D, Barone P,
Rabey M, Bonuccelli U, Forbes A, Breen K, Tluk S, Olanow CW, Thomas S, Rye
D, Hand A, Williams AJ, Ondo W, Chaudhuri KR. Prevalence of nonmotor
symptoms in Parkinson's disease in an international setting; study using
nonmotor symptoms questionnaire in 545 patients. Mov Disord. 2007 Aug
15;22(11):1623-9. http://dx.doi.org/10.1002/mds.21586
[3] Chaudhuri KR, Rojo JM, Schapira AH, Brooks DJ, Stocchi F, Odin P,
Antonini A, Brown RJ, Martinez-Martin P. A proposal for a comprehensive
grading of Parkinson's disease severity combining motor and non-motor
assessments: meeting an unmet need. PLoS One. 2013;8(2):e57221. http://dx.doi.org/10.1371/journal.pone.0057221.
Epub 2013 Feb 27
[4] McNeill A, Duran R, Hughes DA, Mehta A, Schapira AH. A clinical and
family history study of Parkinson's disease in heterozygous
glucocerebrosidase mutation carriers. J Neurol Neurosurg Psychiatry. 2012
Aug;83(8):853-4. http://dx.doi.org/10.1136/jnnp-2012-302402
[5] Gallagher DA, Lees AJ, Schrag A. What are the most important nonmotor
symptoms in patients with Parkinson's disease and are we missing them? Mov
Disord. 2010 Nov 15;25(15):2493-500. http://dx.doi.org/10.1002/mds.23394
[6] Noyce AJ, Bestwick JP, Silveira-Moriyama L, Hawkes CH, Giovannoni G,
Lees AJ, Schrag A. Meta-analysis of early nonmotor features and risk
factors for Parkinson disease. Ann Neurol. 2012 Dec;72(6):893-901. http://dx.doi.org/10.1002/ana.23687
Details of the impact
Parkinson's disease is the second commonest neurodegenerative disease,
now with a lifetime risk in the UK of 4%. Approximately 200,000 people
suffer from PD in the UK (prevalence ~1/300). The treatment of PD is
currently directed to improving the motor symptoms caused by dopamine
deficiency. This is effective in the early stages of the disease, but with
progression, non-motor, non-dopaminergic features such as cognitive,
autonomic and psychiatric disturbances dominate and cause a significant
deterioration of quality of life. Before our work in this area, there was
little recognition of these non-motor features and no means to assess
their prevalence, importance or measure their response to treatment.
Physicians/neurologists in the clinic had no means to evaluate the extent
and severity of this important aspect of PD, and patients had no way to
communicate easily the type, severity and frequency of their non-motor
problems. The scales that we have developed have provided clinicians with
the tools to assess non-motor symptoms and our questionnaires have given
the patient the method to convey the severity of the impact of their
non-motor features on their life. These aspects are crucial in delivering
improved treatment and care to both the patient and more useful
information to the care-giver.
Our scales have now been widely incorporated into clinical practice. For
example, the National Parkinson's Audit Report 2011 noted that many
services were using our questionnaire in preference to one of those
recommended in the Occupational Therapy Best Practice Guidelines [a].
Use of the questionnaire is recommended by Parkinson's UK who have made it
available in print and on their website. They recommend that: "This is
a questionnaire for people with Parkinson's to complete to help health
professionals assess their non-motor Parkinson's symptoms. It should be
completed before visiting your doctor or Parkinson's nurse" [b].
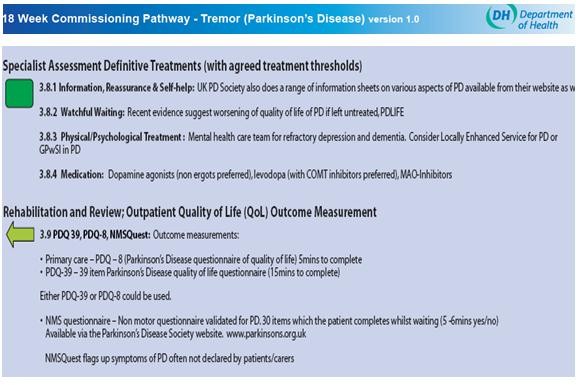
The scale was incorporated into the Department of Health's 18-week
commissioning pathway for Tremor (Parkinson's Disease) which recommended
it as a quality of life outcome measure [c]. It is recommended in
Scottish Guidelines on Diagnosis and pharmacological management of
Parkinson's disease (SIGN 113) [d].
Services treating PD require the questionnaire to be completed in
advance, for example King's College Hospital: "What is required before
referring a patient: For Parkinson's NMSQuest to be completed as per
Department of Health 18-week pathway for Parkinson's (tremor)" [e].
Our clinical services for PD patients at both the Royal Free and UCLH now
routinely use the non-motor questionnaire and scale as part of the
holistic clinical evaluation of Parkinson's disease patients as a means to
design therapeutic strategies at a personal patient level, and to evaluate
the effect of the strategy. We are now working with the Chief Executive of
Parkinson's UK and his team to develop the model for the UK's first `UK
Centre of Excellence' for the management pathway of Parkinson's disease to
include non-motor assessment [f]. This model will then be rolled
out across the UK.
The scales and questionnaires have been adopted by a number of societies
and are widely recommended in guidelines. The International Parkinson and
Movement Disorder Society (an international professional society of
healthcare professionals) provides our questionnaire on their website [g].
The Parkinson Society of Canada has also produced two guides (one for
patients and one for clinicians) which are based on the NMS questionnaire
[h]. The Quality Standards Subcommittee of the American Academy of
Neurology reported that: "The NMS Quest study established a valid and
reliable questionnaire to identify nonmotor symptoms in PD" [i].
The scale is also recommended by the European Parkinson's Disease
Association who say that: "This 30-point questionnaire recognises that
non-movement difficulties often occur in Parkinson's and that it is
important for a doctor to be aware of their extent and the impact they
have on life so that treatment takes these into account. Areas covered
include sleep, Constipation, vision, smell, sexual problems and memory.
The inclusion of such topics in the questionnaire has been found helpful
in opening a dialogue on subjects that might otherwise be ignored or may
be considered to be embarrassing" [j]. The US Parkinson's
Disease Non-Motor Group also provide the questionnaire [k]. The
questionnaire has also been validated for use in other populations and
translated accordingly. It is in use in Japan, North America and many
countries in Europe.
The non-motor questionnaire and scale have recently been incorporated as
secondary endpoints into international Phase II/III clinical trials for
symptomatic treatment in Parkinson disease [l].
Sources to corroborate the impact
[a] National Parkinson's Audit Report 2011:
http://www.parkinsons.org.uk/sites/default/files/parkinsonsaudit_2011report.pdf
see p. 46
[b] Scale available for download from Parkinson's UK: http://www.parkinsons.org.uk/content/non-motor-symptoms-questionnaire
[c] See screenshot at end of this section.
[d] www.sign.ac.uk/pdf/sign113.pdf
See p. 8 and Annex 3.
[e] http://www.kch.nhs.uk/service/a-z/movement-disorders
See tab "Referring to this service"
[f] Can be corroborated by the Chief Executive of Parkinson's UK. Contact
details provided.
[g] http://www.movementdisorders.org/UserFiles/file/NMSS%2030%20items%20revised.pdf
[h] http://www.parkinson.ca/site/c.kgLNIWODKpF/b.8019621/k.2C45/NonMotor_Symptoms_of_Parkinsons_Disease.htm
[i] http://www.neurology.org/content/74/11/924.full
[j] http://www.epda.eu.com/en/parkinsons/in-depth/parkinsonsdisease/rating-scales/pd-nms-questionnaire/
[k] http://www.pdnmg.com/non-motor-symptoms.html
[l] For example: