Scorpion Primers and the Advance of Medical Diagnostics
Submitting Institution
University of SouthamptonUnit of Assessment
ChemistrySummary Impact Type
TechnologicalResearch Subject Area(s)
Biological Sciences: Genetics
Summary of the impact
Research from Professor Tom Brown's group in Chemistry at Southampton has
made a major contribution towards tackling the issue of ineffective drug
therapies using Scorpion Primer Technology. Originally commercialised
through DxS Ltd., the major impacts derived since 2008 include:
- DxS launching, in 2008, its first companion diagnostic product to
allow European sales of Amgen's colorectal cancer drug, Vectibix®;
- The acquisition of DxS by QIAGEN N.V. for US$120 million in 2009;
- FDA approval in 2012 of the KRAS kit for use with the colorectal
cancer drug, Erbitux®;
- FDA approval in 2013 of the EFGR kit for use with the non-small cell
lung cancer drug, Afatinib;
- A 230% increase in QIAGEN staff based at the Manchester site between
2009 and 2012;
- Current annual sales of Scorpion Primer diagnostics are around US$100
million.
Underpinning research
The prescription of ineffectual drug therapies is a recurring problem in
global healthcare, putting patients' lives at risk and wasting vast sums
of health service money. Around 90% of drugs are effective on only 30-50%
of individuals and inappropriate drug treatments can lead to distressing
side effects. A colossal US$350bn of the US$770bn global annual drugs
sales in 2008 was spent on ineffective medicines.
Rapid and accurate analysis of specific DNA sequences can identify groups
of patients that are most likely to respond positively to a particular
drug, thereby enabling improved and cost-effective care. The need to
invent a more effective method of genetic analysis drove a body of
research, from 1998 to 2003, by a team in the University of Southampton
Chemistry Department, led by Tom Brown [3.1, 3.2 ,3.3]. Brown was
a Professor of Biological Chemistry in Southampton Chemistry (until
October 2013) and was also the founder of three biotech companies: Oswel,
ATDBio and PrimerDesign. Brown's group specialises in nucleic acids
chemistry, in particular structure-function relationship, DNA sequence
recognition and the application of oligonucleotide chemistry to genetic
analysis, diagnostics and therapeutics.
The work was conducted in Southampton, mainly by three PhD students under
Brown's supervision: Antonio Solinas, Lynda Brown and Jamie Nicol. In
collaboration with Dr David Whitcombe and Dr Steve Little at AstraZeneca
Diagnostics, the research programme produced an entirely original method
of genetic analysis that was particularly suitable for real-time PCR-based
diagnostics. AstraZeneca and Oswel Research Products, a company
specialising in the synthesis of chemically modified and complex
oligonucleotides and their analogues, DNA, RNA and PNA, funded the
projects jointly. Oswel was founded by Brown and at the time was based in
the University of Southampton.
Brown's primary contribution was to provide the unique chemical expertise
necessary to construct the complex modified oligonucleotides that form the
basis of the technology. Beginning with the analysis of genes involved in
breast cancer susceptibility, the group developed bi-functional molecules
in which a primer is covalently linked to the probe, which is held in a
scorpion-shaped tail (see Figure). The molecules also contain a
fluorophore and a quencher. During the Scorpion polymerase chain reaction
(PCR), in the presence of the target, the fluorophore and the quencher
separate, leading to an increase in the fluorescence emission, which can
be detected and measured in the reaction tube.
Mechanistic studies followed to test the performance of these systems.
Compared with dual-labelled probes, the uni-molecular mechanism acts
around four times faster in solution, for instantaneous fluorescence in
real-time PCR. Its other key advantages, over standard PCR probes that
existed at the time, are enhanced sensitivity, simplified assay design,
high signal-to-noise ratio and greater specificity.
(a)
(b)
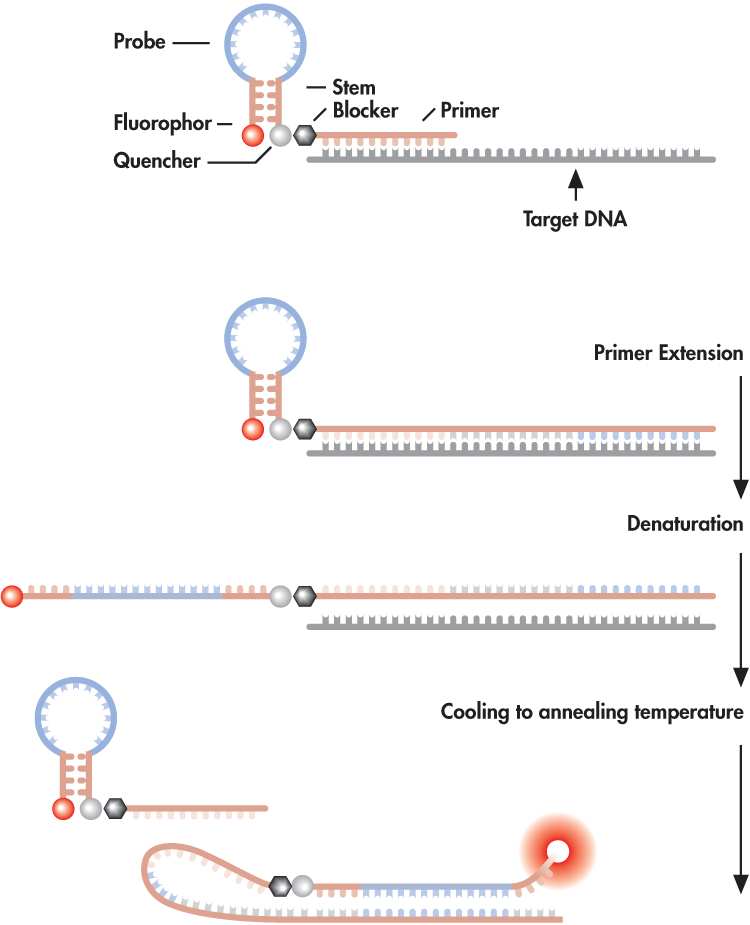 Figure: (a) illustrating the key elements of a Scorpion primer and (b)
how it functions.
Figure: (a) illustrating the key elements of a Scorpion primer and (b)
how it functions.
The research resulted in several highly cited publications, e.g. [3.1]-[3.5].
AstraZeneca filed a US patent for the Scorpion Primers technology
in 1999; a UK patent for "Fluorophore/quencher labelled oligonucleotides"
was filed in the same year.
References to the research
(the best 3 illustrating the quality of work are starred)
Papers:
*[3.1] Whitcombe D., Theaker J., Guy S.P., Brown T., Little S.
`Detection of PCR products using self-probing amplicons and fluorescence'.
Nature Biotechnol. 1999, 17, 804-807
*[3.2] Thelwell N., Millington S., Solinas A., Booth J., Brown T.
`Mode of action and application of Scorpion primers to mutation
detection'. Nucleic Acids Res. 2000, 28,
3752-3761.
*[3.3] Solinas A., Brown L.J., McKeen C., Mellor J.M., Nicol
J.T.G., Thelwell N., et al. `Duplex Scorpion primers in SNP analysis and
FRET applications'. Nucleic Acids Res. 2001, 29,
art. no.-e96.
[3.4] McKeen, C. M.; Brown, L. J.; Nicol, J. T. G.; Mellor, J. M.,
Brown, T. `Synthesis of fluorophore and quencher monomers for use in
Scorpion primers and nucleic acid structural probes'. Org. Biomol.
Chem. 2003, 1, 2267-2275.
[3.5] May, J. P.; Brown, L. J.; van Delft, I.; Thelwell, N.;
Harley, K, Brown. T. `Synthesis and evaluation of a new non-fluorescent
quencher in fluorogenic oligonucleotide probes for real-time PCR'. Org.
Biomol. Chem. 2005, 3, 2534-2542.
Patents filed prior to DxS being founded:
`Methods for detecting target nucleic acid sequences', US patent number
US6326145, Applicant: Zeneca Ltd.; Inventors: D. M. Whitcombe, J. Theaker,
N. J. Gibson and S. Little (filed 1999, granted 2001);
`Fluorophore/quencher labelled oligonucleotides', Applicant: Zeneca Ltd.;
Inventors: N. J. Gibson and T. Brown, UK patent number GB2337992B (filed
1999, granted 2001).
Funding for the underpinning research and studentships was
provided through industrial contributions from the collaborating partner,
AstraZeneca Diagnostics.
Details of the impact
After the development of the new Scorpion Primers technology in
collaboration with Professor Brown (Southampton Chemistry), two scientists
from AstraZeneca (Little and Whitcombe — key contacts) left the company to
set up an SME; DxS Ltd, in Manchester in 2001 (and acquired by
Qiagen in 2009) [5.1, 5.2]. DxS began developing mutation
detection products including an assay for 29 epidermal growth factor
receptor (EGFR) mutations. This led to a range of research products (EGFR,
KRAS, BRAF, PI3K, T315I), a selection of which progressed into TheraScreen
CE-marked diagnostic kits. In January 2008 DxS launched its first
companion diagnostic product to allow European sales of Amgen's
colorectal cancer drug, Vectibix®. Vectibix was initially rejected
by the European Medicines Agency (EMA) on the basis of limited efficacy.
Amgen then used the DxS kit to stratify the population on the basis of
KRAS mutation status and Vectibix was approved by EMA for the KRAS
wild-type population. Further diagnostic products followed, with the EGFR
kit being used to establish the mutation status of non-small cell lung
cancer tumours, to determine likely response to Iressa® or Tarceva®.
DxS was acquired by QIAGEN N.V. for US$120m in 2009 [5.1, 5.2].
QIAGEN, the world-leading provider of assay technologies, is listed on
NASDAQ and the Frankfurt stock exchanges and operates more than 30
subsidiaries in 18 countries. The acquisition of DxS was described by
QIAGEN's CEO as "strategically a highly important transaction" and "a
key element" in its aim to "lead in molecular diagnostic-based
prevention, profiling and personalised healthcare" [5.2].
The sale was recognised as the "Transaction of the Year" (2010) at the
Mediscience Awards, London [5.3]. It also benefited the UK
economy, with QIAGEN establishing a Centre of Excellence in Pharma
Partnering at DxS's Manchester headquarters. The workforce expanded
from 67 (2009) to ~160 (2012) after a move to a purpose-fitted facility
on the Manchester Science Park in 2010. The success of the DxS
assays has led to the growth of QIAGEN's personalized healthcare
portfolio, which now makes US$100 million of sales annually (Peer Schatz,
CEO, QIAGEN N.V.) [5.4].
In the US, Food and Drug Administration (FDA) approval requires
diagnostic assays be associated with a clinical trial. DxS had been
approached by drug companies Amgen, BMS Lilly and Boehringer Ingelheim to
provide companion diagnostic assays for use in their clinical trials. This
led to the approval (July 2012) of the KRAS kit in the US for use with
the colorectal cancer drug, Erbitux® [5.5]. The US market for this
drug is expected to be in the region of US$20 million. The EGFR kit was
submitted to the FDA in November 2012 for use with the Boehringer
Ingelheim non-small cell lung cancer drug, Afatinib; approval was
received in July 2013.
The EGFR and KRAS kits are now being used worldwide by hospital
laboratories, central testing facilities and clinical research
organisations. In the US the KRAS assay is being offered by Lab21, Inc,
The Mayo Clinic, Applied Diagnostics, Inc, Boyce and Bynum Pathology
Laboratories. In January 2013 Clarient also began offering the assay
[5.6]. The CEO of Clarient, Carrie Eglinton Manner, stated: "We
believe precision medicine is the new direction in diagnosing and
treating cancer and Clarient uses state-of-the-art diagnostic
technologies like the therascreen KRAS test to bring clarity and
precision to physicians to assist them in making better treatment
decisions for their patients. Clarient's comprehensive offering and fast
turnaround time coupled with our experience with the therascreen KRAS
test permits us to provide a higher level of performance. Also, the fact
that it is FDA approved provides Clarient with additional assurance of
its quality and reliability." [5.6].
On-going development of the companion diagnostic portfolio is clear from
the agreement between QIAGEN and Eli Lilly, signed in February 2013,
to develop companion diagnostics for drugs for all therapeutic areas of
interest [5.7]. A further expansion of the biomarker portfolio is
evidenced by agreements and licences to develop assays for glioblastoma,
lymphoma and rheumatoid arthritis, in deals with Insight Genetics, Drug
Response Dx, InsermTransfert, Columbia University and the BC Cancer
Agency, Canada [5.4].
Since 2001 Professor Brown has continued to work on Scorpion design with
DxS and later QIAGEN on a consultative basis. Since 2005 this has mainly
been channelled through ATDBio (a spin-out company founded by Tom Brown)
for the provision of specialist advice and custom oligonucleotides [5.8].
David Whitcombe (co-founder of DxS) has stated "Not only was
Professor Brown instrumental in the initial development of Scorpions
(suggesting and supplying appropriate blocking and quenching groups, and
also creating monomer phosphoramidites), but as the technology matured,
he was able to advise and assist on the high quality manufacture of
Scorpion constructs. In addition, his group were highly engaged in the
investigation of the properties and performance of these molecules. This
enabled DxS to move into the manufacture of high quality and high value
Diagnostic tests with significant clinical benefits for large numbers of
cancer sufferers. Ultimately it was the utility and quality
of these tests that underpinned the sale of DxS for US$120m" [5.9].
This is supported by Steve Little (co-founder of DxS), who states that "The
core technology used by DxS was Scorpions and, following the
acquisition, the technology continues to be used in an expanded range of
personalised medicine products provided by QIAGEN. Scorpions
technology now supports annual sales of around US$100m.
The input of Professor Tom Brown in providing chemistry expertise was
central to the success of the Scorpions technology." [5.10].
Sources to corroborate the impact
[5.1] Qiagen company web-site: http://www.qiagen.com/
, main information under section on Molecular Diagnostics: http://www.qiagen.com/Products/Lab-Focus/Molecular-diagnostics
[5.2] Press releases announcing acquisition of DxS by Qiagen: http://www.qiagen.com/About-Us/Press-Releases/PressReleaseView/?PressReleaseID=268&lang=EN;
http://www.genomeweb.com/dxpgx/qiagen-acquires-uks-dxs-deal-worth-130m
[5.3] QIAGEN's acquisition of DxS Ltd. in 2010 was recognized as
the "Transaction of the Year" at the annual Mediscience Awards (London),
the largest annual gathering of publicly quoted healthcare, biotech and
life science companies in Europe: http://www.qiagen.com/About-Us/Who-We-Are/Awards-and-Partnerships/Awards-and-Recognitions/
[5.4] QIAGEN Sales and Expansion of biomarker portfolio: http://www.qiagen.com/About-Us/Press-Releases/PressReleaseView/?PressReleaseID=414&lang=EN
[5.5] Press release on the market size for Erbitux: http://www.genomeweb.com/mdx/fda-clears-qiagen-kras-test-cdx-erbitux
[5.6] Press release announcing Clarient to offer KRAS assay: http://www.qiagen.com/About-Us/Press-Releases/PressReleaseView/?PressReleaseID=404&lang=EN
[5.7] QIAGEN-Eli Lilly agreement: http://www.genomeweb.com/pcrsample-prep/qiagen-eli-lilly-enter-cdx-deal
[5.8] ATDBio web-site: http://www.atdbio.com/
[5.9] Corroborating contact, ex-AstraZeneca collaborator and
co-founder of DxS.
[5.10] Corroborating contact, ex-AstraZeneca collaborator and
co-founder of DxS and ex-Vice President QIAGEN.