Drug Discovery & Clinical Translation
Submitting Institution
University of BathUnit of Assessment
Allied Health Professions, Dentistry, Nursing and PharmacySummary Impact Type
TechnologicalResearch Subject Area(s)
Medical and Health Sciences: Clinical Sciences, Oncology and Carcinogenesis
Summary of the impact
Cancer is a widespread deadly disease; annually, one million new breast
cancers are diagnosed
globally. Endometriosis is a poorly understood disorder, with 80 million
patients worldwide.
Current therapies for both are inadequate and discovery of new drugs is
critical. The Bath group
has pioneered identification of new targets and designed two
"first-in-class" clinical drugs. The
Bath/Imperial College spin-out company Sterix (subsequently acquired by a
major pharmaceutical
company) has translated them into patients and to the pharmaceutical
industry. The steroid
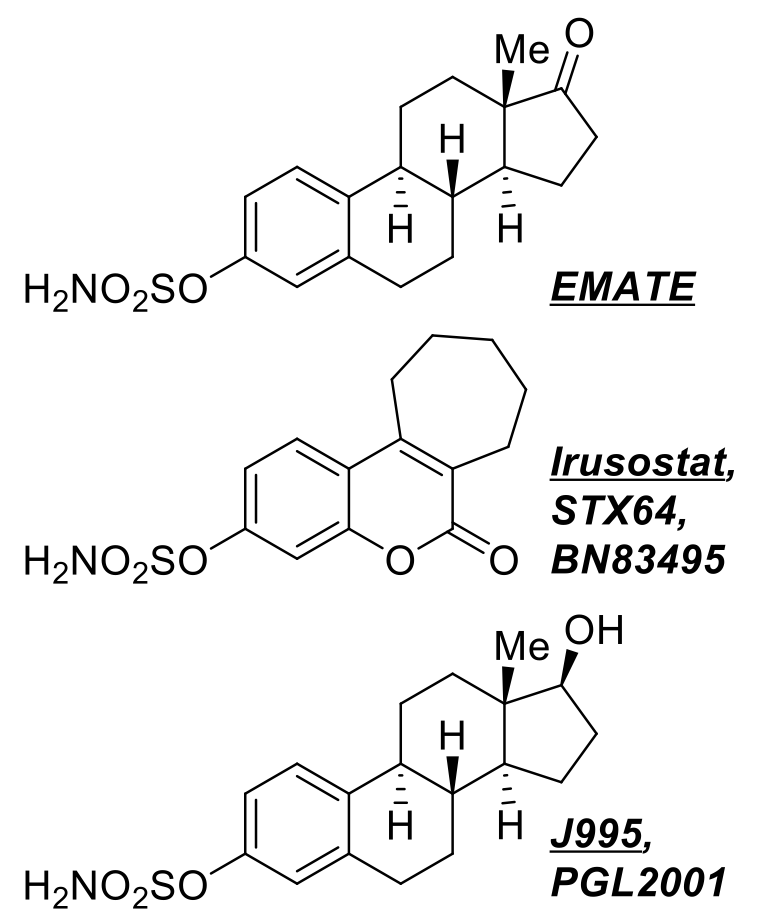
sulfatase inhibitors, Irosustat and J995 have entered
eighteen clinical trials worldwide in patients
with these hormone-dependent diseases, with several ongoing since 2008.
Disease was stabilised
for cancer patients; the advanced clinical evaluation of both drugs is in
progress.
Underpinning research
Basic Science. Many hormone-dependent tumours depend upon
oestrogens for growth and
development. One important source of tumour oestrogen is hydrolysis of
oestrone 3-O-sulfate to
oestrone by steroid sulfatase (STS) [1]. Collaborative research undertaken
at the University of Bath
(led by Professor Barry Potter (1990-date) with Dr LWL Woo (1996-date),
and Dr NM Howarth
(1991-4)) and Imperial College pioneered a novel therapeutic concept to
treat postmenopausal,
hormone-dependent breast cancer through inhibition of STS, and synthesis
of the first highly
potent inhibitors was reported in 1994 [2]. One of these compounds, EMATE,
inhibits the target
irreversibly at picomolar concentrations most probably through an
electrophilic sulfonylamine
generated specifically at the active site of STS. EMATE's
anticancer activity was demonstrated in
vivo at Imperial College. The unprecedented aryl sulfamate
pharmacophore achieved strong patent
protection [3], providing a powerful competitive advantage and supporting
active
commercialisation. Oestrogen sulfamates bind to carbonic anhydrase in red
blood cells and avoid
first-pass metabolism in the liver. Thus, hepatic oestrogenicity is almost
absent, avoiding the over-production
of clotting factors and the associated risk of adverse events typically
seen with
hormone-replacement therapy (HRT) and oral contraception. The
pharmacophore confers
properties that are widely exploitable in drug discovery, therefore;
specifically, excellent oral
activity, bioavailability and pharmacokinetics.
Clinical Translation.
Cancer: Many hundreds of potent non-oestrogenic inhibitors
of STS were also designed at Bath for
applications in oncology [4], supported by Cancer Research UK. This
research led to Irosustat
(also known as STX64) an irreversible STS inhibitor [5] with
excellent oral bioavailability and
pharmacokinetics. CRUK selected Irosustat for the "first-in-class"
Phase I/II clinical trial of an STS
inhibitor in fourteen women with advanced breast cancer
in London and Belfast (2003-2005); it was very well toler-ated
[6]. Median STS inhibition was 98% in biomarker
leucocytes and 99% in target tumour tissue, showing the
effectiveness of the drug, even at 5-20 mg. Strikingly,
five of eight evaluable patients, whose cancer had been
worsening on other therapies [including "third-generation"
aromatase inhibitors], showed evidence of stable disease
for up to 7 months (British Medical Journal; doi:
10.1136/bmj.39213.390243.801);
consequently, some
patients received further compassionate dosing.
Formal academic-industry partnerships between Ipsen (a
French pharmaceutical company), University of Bath and
Imperial College were initiated and drug discovery efforts
were greatly expanded, based upon our very substantial
intellectual property. Joint research demonstrated the
wider applicability of our approach to other hormone-dependent
cancers, e.g., prostate cancer [7] and endometrial cancer [8].
Other diseases: Compound J995 (closely related to EMATE;
also known as PGL2001) started
HRT-related clinical trials in 1998. It has reached Phase II (six clinical
trials to date) with over 170
post-menopausal women being dosed in Phase I and Phase II studies. The
drug is safe and well-tolerated
at all tested doses. Further research between Bath and Imperial
College has also
revealed that Irosustat has potential for treatment of
endometriosis [9].
Recognition of underpinning research. To date, this project has
generated more than one
hundred publications in high profile medicinal chemistry and cancer
journals. Potter has received
several related major academic and industrial prizes. Academic and
translational impact is
underlined by four Royal Society of Chemistry (RSC) medals awarded
since 2007 and the Glaxo-SmithKline
(GSK) International Achievement Award for 2010, relating wholly or in part
to this work.
The citation for the RSC George & Christine Sosnovsky Award &
Medal was "for [Potter's]
landmark contribution to the medicinal chemistry of breast cancer using
a hormone-based
approach and the development of the aryl sulfamate pharmacophore".
The RSC Malcolm
Campbell Memorial Prize and Medal, awarded jointly to the 4-scientist team
from the Bath and
Imperial College was for "Discovery of the first steroid sulfatase
inhibitors and translation into
cancer patients". Importantly, industrial impact was recognised
through the 2010 GSK International
Achievement Award to Potter and Reed (IC), citing "work that has
demonstrated a substantial
advancement in the application of scientific knowledge within the
pharmaceutical sciences". Potter
was denoted 2012 "Investigator of the Year" at the European Life Science
Awards. He is co-inventor
of ca. 770 patent filings worldwide derived from 46 distinct
families. Of these, 430 have
been formally granted, including 45 USPs, 25 EPs and 10 JPs. Illustrative
examples include:
US6653298, US6339079, US6676934 and US6239169.
References to the research
1. Steroid sulfatase: Molecular biology, regulation and inhibition. M J
Reed, A Purohit, L W L
Woo, S P Newman and B V L Potter, Endocrine Rev. (2005) 26,
171-202. DOI:
10.1210/er.2004-0003
2. Estrone sulfamates: potent inhibitors of estrone sulfatase with
therapeutic potential. N M
Howarth, A Purohit, M J Reed and B V L Potter, J. Med. Chem.
(1994) 37, 219-221. DOI:
10.1021/jm00028a002
3. Steroid Sulfatase Inhibitors. M J Reed and B V L Potter, US Patent
5,616,574 (1997).
https://docs.google.com/viewer?url=patentimages.storage.googleapis.com/pdfs/US5616574.pdf
4. Structure-activity relationship for the first-in-class clinical
steroid sulfatase inhibitor Irosustat
(STX64, BN83495). L W L Woo, D Ganeshapillai, M P Thomas,
O B Sutcliffe, B Malini, M F
Mahon, A Purohit and B V L Potter, ChemMedChem (2011) 6,
2109-2034 [recognised as VIP
paper with journal front cover feature]. DOI: 10.1002/cmdc.201100288
5. Potent active site-directed inhibition of steroid sulphatase by
tricyclic coumarin-based
sulphamates. L W L Woo, A Purohit, B Malini, M J Reed and B V L Potter, Chem.
& Biol.
(2000) 7, 773-791. DOI: 10.1016/S1074-5521(00)00023-5
6. Phase I study of STX64 (667 Coumate) in breast cancer patients: the
first study of a steroid
sulfatase inhibitor. S Stanway, A Purohit, L W L Woo, S Sufi, D Vigushin,
R Ward, R Wilson, F
Z Stanczyk, N Dobbs, E Kulinskaya, M Elliott, B V L Potter, M J Reed and R
C Coombes, Clin.
Cancer Res. (2006) 12, 1585-1592. DOI:
10.1158/1078-0432.CCR-05-1996
7. The development of steroid sulfatase inhibitors for hormone-dependent
cancer therapy. J M
Day, A Purohit, H J Tutill, P A Foster, L W L Woo, B V L Potter and M J
Reed, Ann. N.Y. Acad.
Sci. (2009) 1155, 80-87. DOI:
10.1111/j.1749-6632.2008.03677.x
8. The use of steroid sulfatase inhibitors as a novel therapeutic
strategy against hormone
dependent endometrial cancer. P A Foster, L W L Woo, B V L Potter, M J
Reed and A Purohit,
Endocrinology (2008) 149, 4035-4032. DOI:
10.1210/en.2008-0223
9. Inhibition of steroid sulphatase activity in endometriotic implants by
667 COUMATE: a potential
new therapy. A Purohit, L Fusi, J Brosens, D Parish, M S Fernandes, L W L
Woo, B V L Potter
and M J Reed. Human Reproduction (2008) 23, 290-297. DOI:
10.1093/humrep/dem308
Details of the impact
Economic underpinning that has enabled clinical impact since 2008:
Initial clinical translation and
£1.8M revenue was achieved through a licence to a major international
pharmaceutical company
for EMATE as a synthetic liver-sparing oestrogen, the first since
ethinylestradiol (first marketed in
1938) suitable for oral dosing. This licence funded, in 1998,
incorporation of the Bath-Imperial spin-out,
Sterix. Intellectual property was vested with Sterix from which large
development contracts to
both universities [ca. £12M to Bath (1998-2010)] facilitated
R&D activity that led to Irosustat. Sterix
attracted £8M of venture capital in 2001 to support the initial clinical
trial of this drug. Then, in
2004, Sterix was acquired by the French pharmaceutical company, Ipsen,
which provided
substantial research funding to both universities [£8.3M to Bath] and
initiated wider clinical trials.
Revenue for J995 from initial licensing and milestones from
1998 through to 2003/4 in Europe
amounted to £4.2M and a Japanese licence of £1.34M was also secured during
2002-2003 [Sterix
Ltd, Annual Accounts, Companies House]. Sterix employed up to 40 research
staff, directly or
indirectly, and returned ~£28M to the UK university sector in direct
research contracts.
Overview: Successful drug discovery in academia is a very rare
event and clinical translation is
even rarer. The validation of new drug targets, the synthesis of
"first-in-class" drugs to address
them, and their clinical translation into humans reflects extraordinary
impact by any measure.
Moreover, this success has been achieved twice, dosing hundreds of healthy
women volunteers as
well as cancer patients with drugs first designed at Bath. Overall, this
research has had impact for
industry, the economy, clinical practitioners and, most importantly,
patients. There is also potential
for further benefit deriving from ongoing clinical trials. The work has
facilitated the design of
compounds with highly desirable pharmaceutical properties and has defined
novel therapies with
indicative clinical proof of benefits to patients primarily in
hormone-dependent cancers.
Breast cancer: The first clinical trial (2003-05) of Irosustat
demonstrated encouragingly positive
effects in breast cancer patients and, after the acquisition of Sterix,
Ipsen continued development
of both Irosustat [STX64, BN83495] and J995
[PGL2001] in concert with the two universities. Ipsen
refined the pre-clinical package for Irosustat to full industrial
standard. New international clinical
trials have commenced since 2008 for Irosustat in breast cancer
[1], prostate cancer and
endometrial cancer [2], initiated by Ipsen. Concomitantly, Ipsen published
in late 2009 [1] the
clinical observation of stable disease in a new cohort of thirty-five
oestrogen receptor-positive
metastatic breast cancer patients, after dose optimisation [3].
Importantly, in this trial, the disease
of one patient was stable for thirteen months, one for eight months, one
for seven months and
three for ca. six months. Biopsy-validated erythematous skin
infiltration in one patient was no
longer visible after one month of treatment. Nearly complete inhibition of
the target enzyme was
observed at all doses. Significantly, the Director of the Imperial CRUK
Cancer Centre, the clinician
who led the study, said of the effects on patients: "To date, four of
the patients who received
Irosustat [BN83495] had tumours that remained stable for at least 6
months. One of these had
cutaneous metastases that improved after one month of treatment. This is
very encouraging, as
these women are patients who are reaching the end of their hormonal
treatment options.
Importantly, Irosustat [BN83495] was well tolerated at the selected
dose." He added: "I am
confident that Irosustat [BN83495] will become a new hormonal option in
the treatment of post-menopausal
women with oestrogen receptor-positive metastatic breast cancer"
[3].
Further impact is illustrated by the formal use of this work (published
in The Oncologist, a journal
for practising clinical oncologists,) in a Continuing Medical
Education (CME) programme for
"physicians who wish to advance their current knowledge of clinical cancer
medicine in breast
cancer and are involved in providing patient care in a cancer care
environment" [4]. Finally,
overseen and substantially funded by CRUK, two further clinical trials
with Irosustat were initiated
in 2012; these trials aim to explore the benefit of combination dosing
with an aromatase inhibitor
and also to examine the effects of Irosustat in breast cancer
patients using PET scanning [5,6].
Other cancers: Drug discovery research at Bath has also provided
the stimulus for active clinical
trials against other cancers: androgen-dependent prostate cancer and
endometrial cancer inter
alia. Prostate cancer represents a large unmet medical need and a
Phase I/II clinical study [17
patients] of Irosustat commenced in 2008 at three centres in the
USA (Johns Hopkins, Duke and
Wisconsin), evaluating the pharmacodynamics and safety of the drug in
metastatic prostate cancer
patients [7]. A Phase II clinical programme comparing Irosustat
and megestrol acetate in recurrent
or metastatic advanced post-menopausal endometrial cancer patients at 44
separate centres
worldwide started in 2009. Results to-date have shown inter alia
significant stable disease (47%)
and an advantageous safety profile for Irosustat [8].
Endometriosis: Since 2008, clinical trials have also taken place
in endometriosis through PregLem
(Switzerland) and Gedeon Richter (Hungary). Ipsen spun out PregLem in 2007
with one of its two
main clinical assets being J995 [PGL2001], licensed outside
oncological indications. Endometriosis
is a benign gynaecological disease characterised by the presence of
endometrial tissue outside the
uterus, leading to chronic pelvic pain and infertility. In addition to
production of oestrogens in the
ovaries, there is compelling evidence that local synthesis of oestrogens
in endometriotic lesions
promotes progression of the disease and resistance to endocrine therapy.
With an estimated 80M
patients worldwide, the disease is still poorly understood, most
treatments have unpleasant side-effects
and current therapies are grossly inadequate. J995 [PGL2001],
is en route to be the first of
a new class of treatment for endometriosis and other benign gynaecological
conditions, and
entered clinical trials in Germany in 2008 in healthy pre-menopausal women
[9] to advance this
compound towards a novel, once-a-week, oral medication. J995 [PGL2001]
was part of the clinical
assets of PregLem, acquired by the Hungarian drug company Gedeon Richter
in 2010 in a deal
valued at ca. €337M [10]. The drug continues in clinical trials
against endometriosis and
multicentre Phase IIa studies to investigate its efficacy, safety,
pharmacokinetics and
pharmacodynamics started in Hungary, Poland and Romania in 2012 [11].
Sources to corroborate the impact
- Ipsen clinical trial results published at the AACR San Antonio Breast
Cancer Conference 2009:
A Phase I Dose Escalation Study of Steroid Sulfatase Inhibitor
BN83495/STX64 in
Postmenopausal Women with ER Positive Breast Cancer: R Coombes, et
al., Cancer Res.
2009, 69 (suppl. 24), 4097.
http://cancerres.aacrjournals.org/cgi/content/abstract/69/24_MeetingAbstracts/4097
- Ipsen initiates an Advanced Endometrial Cancer program with BN83495,
its first-in-class
steroid sulfatase (STS) inhibitor. http://www.ipsen.com/wp-content/uploads/2013/03/20091125___bn83495_phase_ii_10.pdf
- Ipsen establishes optimal biological dose for BN83495 steroid
sulphatase (STS) inhibitor in
oestrogen receptor-positive metastatic breast cancer. http://www.ipsen.com/wp-content/uploads/2013/03/PR-BN83495-Breast-cancer-Phase-I-EN-FINAL.pdf
- Work used for Continuing Medical Education (CME) programme during 2008
in major clinical
translational oncology journal: Steroid sulfatase: a new target for the
endocrine therapy of
breast cancer. SJ Stanway, et al., The Oncologist (2007) 12,
370-374.
http://theoncologist.alphamedpress.org/content/12/4/370.full.pdf+html
- In 2012, one of two CRUK-supported combination clinical trials of
STX64 with an aromatase
inhibitor commenced — the IRIS trial: http://www.cancerresearchuk.org/cancerhelp/trials/a-study-looking-irosustat-treat-advanced-breast-cancer-iris
and
http://public.ukcrn.org.uk/Search/StudyDetail.aspx?StudyID=12479
- The IPET trial, in which the effects of Irosustat on breast cancer
will be evaluated by Positron
Emission Tomography scanning: http://clinicaltrials.gov/show/NCT01662726
- BN83495 in Prostate Cancer (STX64PC): http://clinicaltrials.gov/show/NCT00790374
- A Phase II multicentre randomized open-label study of oral steriod
sulphatase (STS) inhibitor
Irosustat (BN83495) versus megestrol acetate (MA) is women with advanced
/ recurrent
endometrial cancer (EC). P Pautier, et al., Annals Oncol. (2012)
23 (suppl. 9) 329.
http://www.cancer.gov/clinicaltrials/search/view?cdrid=644880&version=HealthProfessional&pr
otocolsearchid=6996601
- Preglem SA initiates Phase 1b clinical trial for its steroid sulfatase
inhibitor PGL2001.
http://www.preglem.com/sites/default/files/news/2012-01-17/2008-03-12-%20PR%20PregLem-%20SAPHIR%20study-%20Phase%20Ib%20Clinical%20Trial.pdf
- Richter announces the acquisition of Preglem. http://www.richter.hu/EN/Pages/pr101007.aspx
http://preglem-com.preglem-cloud.com/en/research-and-development
- PGL2001 Proof of Concept Study in Symptomatic Endometriosis: (AMBER)
http://clinicaltrials.gov/ct2/show/NCT01631981?term=steroid+sulfatase&rank=4