CHEM04 - Ozone-depleting halogens in the atmosphere
Submitting Institution
University of YorkUnit of Assessment
ChemistrySummary Impact Type
PoliticalResearch Subject Area(s)
Chemical Sciences: Physical Chemistry (incl. Structural), Other Chemical Sciences
Summary of the impact
The international Montreal Protocol limits the production of
stratospheric-ozone depleting
substances that contain chlorine and bromine. York researchers used the
Atmospheric Chemistry
Experiment (ACE) satellite to monitor the decay of halogen-containing
molecules in the
stratosphere and to re-evaluate their atmospheric lifetimes. This York
research also determined
that oceans represent a vast reservoir of organohalogens, which are
released to air and impact
significantly on ozone destruction. The research results have been
incorporated into the
conclusions of the World Meteorological Organization/United Nations
Environment Programme
(WMO/UNEP) Scientific Assessments on Ozone Depletion, the pre-eminent
knowledge base used
for international policy and domestic legislation. Experimental
infrastructure created in this York
research now contributes to UK Government obligations under the United
Nations Framework
Convention on Climate Change (UNFCCC) and informs it of long-term
atmospheric change.
Underpinning research
Stratospheric ozone protects humankind and its crops from harmful
ultraviolet radiation.
Anthropogenic stratospheric ozone destruction is caused mainly by
long-lived chlorine-containing
molecules such as CCl4 and chlorofluorocarbons (CFCs), and
bromine molecules such as halons
(brominated fluorocarbons) and methyl bromide. The 1987 Montreal Protocol
on substances that
deplete the ozone layer and its subsequent amendments have resulted in the
phasing-out of the
production of many such chlorine-and bromine-containing species —
preventing not only millions of
cases of skin cancer deaths and cataracts, but also reducing emissions of
halogenated
greenhouse gases by the equivalent of more than 10 billion tonnes of CO2
at the end of 2008. In
view of the steady progress made under the Protocol, former UN
Secretary-General Kofi Annan
called it in 2003 Perhaps the single most successful international
agreement to date
(ozone.unep.org/Publications/MP_Key_Achievements-E.pdf).
It is crucial to assess the performance of the Montreal Protocol in
reducing the concentrations of
ozone-depleting substances (ODSs) and to monitor the recovery of the ozone
layer. Therefore,
every four years the WMO and UNEP publish a report on the ``Scientific
Assessment of Ozone
Depletion'', carried out by the Scientific Assessment Panel (SAP). The
York-based group, led by
Bernath, contributed to the WMO ozone report by measuring global
concentration distributions of
many chlorine-containing molecules using data from the ACE satellite1
(www.ace.uwaterloo.ca).
- UV photodissociation of these molecules forms intermediates such as
phosgene (Cl2CO) and
ClFCO 1,2 and leads ultimately to the formation of HCl. ACE
satellite measurements tested the
accuracy of chemical models and showed the anticipated decline in HCl
and other halogenated
gases due to the implementation of the Montreal Protocol.
- Atmospheric lifetimes are crucial parameters in models and the
research group determined the
relative stratospheric lifetime of CCl4 by the method of
tracer-tracer correlations; there are
surprisingly large uncertainties in these lifetimes (see 2010 WMO ozone
report).
- CCl4 is destroyed mainly by photolysis in the stratosphere
giving phosgene. Fu et al. measured
the first global distribution of phosgene2 and found that
concentrations had declined from earlier
in situ measurements owing to decline in the parent source gases
CH3CCl3 and CCl4.
- The York group found that the global distribution of FClCO,1
produced by photolysis of the
parent CFC-11 (CF3Cl) molecule, agreed well with that
calculated by an atmospheric model,
giving confidence in the reliability of the emissions projections and
the model.
Conventionally, it was thought that anthropogenic bromine compounds in
the form of halons and
CH3Br were the sole carriers of bromine into the stratosphere.
However, calculated stratospheric
Bry concentrations from measurements of BrO were too
high by 15-40% to be explained by these
halocarbons alone. The oceanic and atmospheric measurements of Carpenter
in York showed that
emissions of natural bromine compounds including CHBr3 and CH2Br2
from the marine biosphere,
especially macroalgae, represent a major global source of bromine.3
Subsequent model studies
showed that these emissions were sufficient to explain the discrepancy
between modelled and
measured stratospheric BrO concentrations. The York group demonstrated
that ozone in the
troposphere was also controlled over wide oceanic areas by natural halogen
emissions,4 and this
has led to public and policy debate on the potential contribution of such
processes to the reduction
of ground level ozone, a key pollutant (in contrast to the essential
nature of stratospheric ozone).
Both the ACE and the tropospheric research are part of multi-centre
programmes involving
collaboration of York with other institutes in Canada, USA, Belgium, UK,
Germany and France.
Key researchers:
Peter F. Bernath: Appointed 01/07/2006 as Professor,
Lucy J. Carpenter: Appointed 01/09/2000 as Lecturer A, Promotion to Chair
01/10/09
References to the research
This research exceeds the quality threshold as is evident from the
journal quality, the number of
citations (citation data from Scopus, November 2013) and recognition in
prizes.
Peer-reviewed publications -- Authors at York in bold
1. D. Fu, C. D. Boone, P. F. Bernath, D. K. Weisenstein, C. P.
Rinsland, G. L. Manney, and K. A.
Walker, First global observations of atmospheric COClF from the
Atmospheric Chemistry
Experiment mission, J. Quant. Spectrosc. Radiat. Transfer, 2009, 110,
974. DOI:
10.1016/j.jqsrt.2009.02.018. 5 citations
2. D. Fu, C. D. Boone, P. F. Bernath, K. A. Walker, R. Nassar, G.
L. Manney, and S. D. McLeod,
Global phosgene observations from the Atmospheric Chemistry Experiment
(ACE) mission,
Geophys. Res. Lett., 2007 34, L17815. DOI:
10.1029/2007GL029942. 7 citations
3. L. J. Carpenter, P. S. Liss and S. A. Penkett, Marine
organohalogens in the atmosphere over
the Atlantic and Southern Oceans, J. Geophys. Res.- Atm., 2003, 108,
DOI:
10.1029/2002JD002769. 49 citations
4. K. A. Read, , A. S. Mahajan, L. J. Carpenter, M.
J. Evans, B. V. E. Faria, D. E. Heard, J. R.
Hopkins, J. D. Lee, S. J. Moller, A. C. Lewis,
L. Mendes, J. B. McQuaid, H. Oetjen, A. Saiz-Lopez,
M.J. Pilling, and J. M. C. Plane, Extensive halogen-mediated ozone
destruction over the
tropical Atlantic Ocean, Nature, 2008, 453, 1232. DOI:
10.1038/nature07035. 133 citations
Other evidence of quality:
2012 Benedict Spectroscopy Award to P. Bernath: (from Elsevier Press on
behalf of the Journal of
Quantitative Spectroscopy and Radiative Transfer) The citation
states that Bernath "has also been
at the forefront of satellite remote sensing experiments, particularly as
the mission scientist for the
ACE (Atmospheric Chemistry Experiment) satellite"
2009 Alouette Award of Canadian Aeronautics and Space Institute (CASI) to
P. Bernath for
developing the ACE satellite and the resulting observations (shared with
V. Wehrle, G. Rumbold, I.
Walkty, T. McElroy and M.-A. Soucy)
2006 Philip Leverhulme prize to L. J. Carpenter — citation: "Not only has
she made pioneering
measurements [on the chemistry of atmospheric bromine and iodine
compounds], but she has also
contributed substantially to the interpretation of the observations to
shed light on the underlying
chemistry. These results are of great significance in understanding the
Earth's climate."
Selected research grants:
Bernath, 2008-2011, 'VOCs in the Troposphere Retrieved from ACE Satellite
Measurements',
NERC, £364,058.
Bernath, 2011-2013, 'Satellite Observations of Halogen-Containing
Molecules', NERC, £349,740.
Carpenter and Lewis, 2005-2008, 'UK SOLAS Atmospheric Observatory at Cape
Verde', NERC
SOLAS contract, £572,220.
Carpenter, 2006-2009, Philip Leverhulme Prize in `Earth Ocean and
Atmospheric Sciences',
Leverhulme Trust, £70,000.
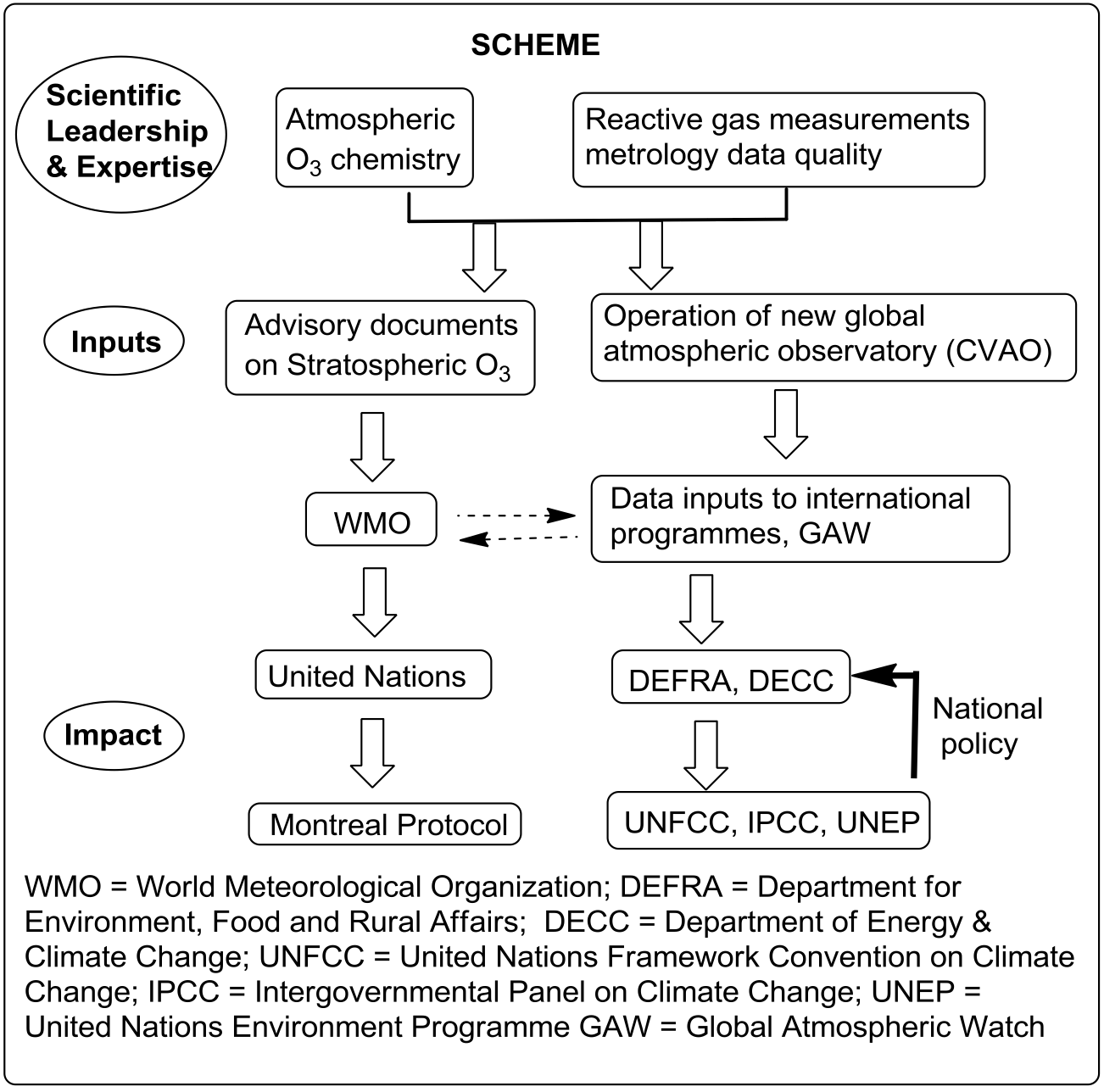
Details of the impact
The York research created impact by: (a) measuring changes in
stratospheric atmospheric
composition and determining lifetimes of ozone-depleting substances; these
results are needed by
UNEP in order to make decisions, especially to provide evidence of policy
effectiveness and a
more robust prediction of the likely timescales for ozone hole recovery;
(b) revealing natural
halogenated emissions that close the gap between modelled and measured
stratospheric BrO
concentrations, providing confidence in the models used to inform UNEP of
stratospheric halogen
changes; (c) by serving on the Scientific Assessment Panel (SAP) that
advises UNEP; (d) by
proving the value to the UK of long-term background tropospheric
measurements of trace gases
and enabling the UK to fulfil its international obligations.
The SAP is charged with the periodic updating of the scientific
understanding of the depletion of
stratospheric ozone as summarised by A.R. Ravishankara (co-Chair of SAP). "The assessment is
written and reviewed by leading
experts in the international
atmospheric sciences
community at the request of the
Parties to the U.N. Montreal
Protocol [197 countries]. It
forms the scientific basis for
decisions that the Parties reach
with regard to the phaseout of
ozone-depleting chemicals such
as the CFCs and other matters
related to the long-term
protection of the stratospheric
ozone layer. As you may know,
all previous Montreal Protocol
decisions have been based on
the science findings of the
Scientific Assessment Panel....
For previous assessments, it
has proven true that the
participants have found their
efforts rewarded with increased
stature and recognition in the
scientific community. Likewise,
their supporting institutions
have received recognition for their leadership in providing world-class
scientific expertise to the
international effort."5 The Assessment process
involves scientific research and stakeholders
including governments and their agencies, research managers, industries
and the public.
The 2010 Ozone Assessment,6 and two earlier reports, make
significant reference to the original
underpinning research undertaken in York, which contributed to a better
understanding of the
observed behaviour of ODSs and ozone in the stratosphere and thus to
continued international
acceptance of the Montreal Protocol. Bernath and Carpenter also contributed
substantially as both
reviewers of the 2010 report and members of the 2010 Scientific Assessment
Panel.
York impact on Scientific Assessment Panel — stratospheric ozone.
The projections of different
ozone depleting gases restricted by the Montreal Protocol including CCl4,
CH3CCl3 and CFC-11,
and their photochemical products have been evaluated through the ACE
measurements.
- In the 2010 report6 it was noted that CCl4
declined more slowly than predicted in the 2006
report; these predictions are very sensitive to the assumed atmospheric
lifetime. The new
stratospheric lifetime contributed to the assessment that the problem is
due to unreported
emissions, rather than an erroneous lifetime.
- The end product of the destruction of chlorinated gases in the
stratosphere and lower
mesosphere is HCl. Observations of HCl reduction in the lower mesosphere
by ACE contributed
to the overall assessment of the success of the Montreal Protocol in the
2010 report.6
- In relation to natural brominated compounds, the research provided
emissions estimates,
hitherto unidentified species, and new understanding of the
halogen composition and chemistry
of the stratosphereand troposphere.3,4 The major
impact is that inclusion of such natural
bromine species and their chemistry in global models has led to better
agreement of the model
with inorganic bromine measurements in the stratosphere, as detailed in
the 2010 report.6 This
provides evidence that the policy is effective and improves the robust
prediction of the
timescales for ozone hole recovery.
York impact — tropospheric ozone. Whilst the role of halogen
chemistry in controlling stratospheric
ozone has been well known for several decades, the impact on
tropospheric ozone has been less
understood. Ozone in the troposphere is important for several reasons;
it is an air pollutant, a
greenhouse gas in its own right, and indirectly it controls the lifetime
of methane, another important
greenhouse gas. The roles of both ozone and methane as greenhouse gases
are increasing in
importance. The new knowledge of halogen-tropospheric ozone processes
reported by the York
scientists4 has contributed to policy recommendations; the
Royal Society Ozone report (2009)7
used the research to highlight the need to understand and control
background ozone
concentrations in relation to air quality. The research was also
reported widely in the media and
led to public debate on the potential of natural processes to mitigate
against air pollution.8
The research programme4 has achieved long-term impacts
through the building of experimental
infrastructure. The proposal for a tropical ocean atmospheric
observatory was driven by Carpenter
(York) with Leeds colleagues and resulted in the construction of an
observatory in Cape Verde
(www.ncas.ac.uk/cvao). The
research demonstrated the value of long-term monitoring of the
tropical atmosphere.4 As a result, the observatory is now
supported as a permanent UK
contribution to the World WMO Global Atmospheric Watch programme (www.wmo.int/gaw);
it is
one of 28 stations worldwide and the only station supported by the UK
Government. It employs two
permanent Cape Verdean technicians (one of whom is now enrolled on an
MSc in Chemistry at
York) as well as UK researchers. The data generated from it now form
part of the UK capability to
meet its obligations on climate change detection under UNFCCC. The
research continues to
generate long-term observations of trends in ozone and other gases, and
these data are
disseminated to academic and Government users via agencies such
as the Department of Energy
and Climate Change (DECC), UNFCCC and WMO. The data alerts the
government to changes in
the Atlantic atmosphere arising from pollution episodes or long-term
changes.
Quotation from L. Jalkanen Chief, Atmospheric Environment Research
Division, World
Meteorological Organization,9 "The University of York,
with support from other UK and German and
Cape Verdean partners, created in 2006 a new observatory in Cape
Verde. This observatory
helped GAW fill in an important gap in global observations, providing
information on changes
occurring in the tropical North Atlantic marine boundary layer, a
region sensitive to both natural and
anthropogenic change. ... The global reach of the GAW programme, and
its contributor scientists,
allows it to also influence governmental understanding and policy on
atmospheric change.
Sources to corroborate the impact
Reports related to policy
- Letter from Co-chair Montreal Protocol Scientific Assessment Panel
- WMO/UNEP Assessment Report on Stratospheric Ozone 2010, Chapter 1
Ozone-Depleting
Substances (ODSs) and Related Chemicals,
http://ozone.unep.org/Assessment_Panels/SAP/Scientific_Assessment_2010/00-SAP-2010-Assement-report.pdf
- Royal Society report "Ground-level ozone in the 21st century: future
trends, impacts and policy
implications" 2008. http://royalsociety.org/policy/publications/2008/ground-level-ozone
References to public debate (not exhaustive) relating to reference
4:
- BBC World Service interview given by Prof L J Carpenter (26.06.08,
Science In Action, "Low
level ozone pollution". http://worldservice.prototyping.bbc.co.uk/programmes/X0901226
(sign up
required); The Guardian newspaper online
(http://www.guardian.co.uk/environment/2008/jun/26/climatechange.pollution?INTCMP=SRCH);
New Scientist Online (http://www.newscientist.com/article/dn14211-tropical-ocean-sucks-up-vast-amounts-of-ozone.html);
A solicited Nature News and Views article (von Glasow, Nature,
2008,
453, 1195-1196), Nature Reports Climate Change (Newton,
"A natural detox", DOI:
10.038/climate.2008.67)
- Letter from Chief, Atmospheric Environment Research Division, WMO,
Geneva
Page 4