CS2 - The development of low-cost point of care sensors for the detection of protease enzymes
Submitting Institution
Queen Mary, University of LondonUnit of Assessment
ChemistrySummary Impact Type
TechnologicalResearch Subject Area(s)
Biological Sciences: Biochemistry and Cell Biology
Medical and Health Sciences: Dentistry, Pharmacology and Pharmaceutical Sciences
Summary of the impact
The spin-out company, Degrasense, has developed and protected
intellectual property of technology capable of quantifying specific
proteolytic enzymes through changes in electrochemical responses
(impedance) at electrodes due to the enzymatic degradation of polymer
coatings. The company has detected several specific proteases that are
relevant to the monitoring and treatment of a number of conditions
including: periodontal disease, multiple sclerosis, haemophilia and
hypertension. The technology is currently being validated in a clinical
trial as a point of care sensor for the detection of active periodontal
disease. Point-of-care sensors provide immediate, low-cost test results in
non-laboratory settings, offering a more patient-centred approach to
healthcare and earlier detection of disease.
Underpinning research
Staff leading the underpinning research
Dr Steffi Krause (School of Engineering and Materials Science) and
Professor Michael Watkinson (School of Biological and Chemical Sciences)
have been collaborating since 2006 on the development of new, generic, low
cost and non-invasive sensor arrays for the specific detection and
quantitation of a number of proteolytic enzymes, which are clinically
relevant markers for a number of disease states. They have established the
spin-out company Degrasense, which owns the underpinning intellectual
property and are developing the technology via clinical trials.
Other notable QMUL researchers working with Watkinson and Krause include
Dr Jacqueline Stair (BBSRC-funded PDRA, 2008-2009), Dr Xingewi Zheng
(IP2IPO Ltd-funded PDRA, 2008-2010) and Dr Joseph Cook (Technology
Strategy Board-funded PDRA, 2008-2009).
Research underpinning the new technology
The technology is based on the preparation of hydrogel polymers that are
cross-linked with short peptide sequences containing amino acid sequences
cleaved by the specific proteolytic enzymes being targeted [references
1-4].
Through BBSRC-funded research, Krause, Watkinson and Stair developed new
peptide cross- linked dextran hydrogels, which, they demonstrated using a
quartz crystal micro-balance, can sense protease cleavage [see publication
1]. The underpinning work involved the preparation of suitably
functionalised peptides, which could be used to cross-link dextran to form
degradable hydrogel polymers. The key synthetic challenges were to
identify both the functional group which could be cross-linked and the
nature of the polymeric matrix which would allow degradation by the
individual protease in question, at clinically relevant concentrations and
within a short time-frame, without non-specific protein binding. Several
approaches were investigated including the in situ radical
polymerisation of peptides and the cross-linking of highly functionalised
dendronised polymers. Ultimately it was found that a partially oxidised
dextran in combination with amine-functionalised peptides provided
suitable substrates which met the technical specification required.
Consequently they were able to coat individual electrodes with different
polymers so that each electrode is able to detect a specific enzyme. This
aspect of the research is central to the technology — without it no point
of care device could have been produced.
In a second publication [2], Watkinson, Krause, Zheng and Cook
demonstrated the efficacy of this technology in the selective detection of
a number of proteases (Human Neutrophil Elastase, Cathepsin-G, and MMP8)
that are clinically relevant to periodontal disease. Periodontal disease
(periodontitis) is an inflammatory disease that affects the periodontum,
the tissues that surround and support the teeth. If left untreated,
chronic periodontitis leads to degradation of bone and ultimately to the
loss of teeth. However, progression of the disease is not uniform across
infected sites and accurate clinical methods for distinguishing areas
where the disease is active, rather than inert, are currently lacking. As
a result much unnecessary treatment of periodontal sites that are
quiescent occurs. This is both costly and potentially causes additional
damage to teeth attachments.
The research, led by Krause and Watkinson [1, 2], clearly established the
potential for a new technology, as a point-of-care clinical diagnostic
tool of protease activity associated with periodontal disease. The
intellectual property underpinning the technology was protected by a
European patent [3] and a further patent application has been filed [4].
The potential of this technology led to follow-on funding secured from
the BBSRC and the Heptagon fund in 2006. Then with support from the
Technology Strategy Board, and in collaboration with a number of key
industrial partners, Watkinson and Krause were able to develop the
technology to the point where it could be tested in a clinical trial. They
have also secured Venture Capital (VC) funding and, most recently, funding
from the Barts and the London Charity.
References to the research
Peer Reviewed Papers
1. J. Stair, M. Watkinson and S. Krause, "A generic protease sensor
material based on the degradation of peptide cross-linked dextran
hydrogels", Biosensors and Bioelectronics, 2009, 24, 2113-2118.
2. X. Zheng, J. Cook, S. Yang, S. Krause, M. Watkinson, I. Douglas and A.
Rawlinson, "Generic protease detection technology for monitoring
periodontal disease", Faraday Disc., 2011,149, 37-47.
Patents
3. S. Krause, D. Kamarun, M. Watkinson and J. Stair, "Sensor coatings for
protease detection", European patent number 07824180.9-1223
PCT/GB2007/003929, granted 10.06.2009 currently at the national phase in
the US (WO2008047095(A1)) and the regional phase in Europe
(EP2082057(A1)).
4. S. Krause, X. Zheng, and M. Watkinson, A second UK priority
application following on from Patent 1 was filed on July 30th 2010 and is
entitled "Sensor Coating Layer, Device and Method" (App No. 1012902.1).
Funding
£124,876 to G. Giovannoni (PI), S. Krause (co-I), M. Watkinson (co-I), D.
Baker (co-I) and A Nassim (co-I) for a project entitled "MMP-9 detector
for inflammation monitoring in autoimmune diseases and solid organ graft
rejection" (NSCG1L3R), January 2012-December 2014.
£738,583 EPSRC/DTI Technology Strategy Board to S. Krause (PI) and M.
Watkinson (co-I) for a project entitled "Prototype sensor for periodontal
disease monitoring" (TP/8/BIO/6/I/Q0020H) April 2008-.December 2011
£18,952 Queen Mary Innovations Ltd to M. Watkinson (PI) and S. Krause
(co-I) for a project entitled "Sensor Platform for the Detection of
Coagulation Factors" December 2010-March 2011.
£109,710 BBSRC follow-on fund awarded to S. Krause (PI) and M. Watkinson
(co-I) for a project entitled "Disposable low cost sensor for periodontal
disease", BB/E525877/1 (co-investigator) April 2006-March 2008.
£95,839 Heptagon Fund awarded to S. Krause (PI) for a project entitled
"Disposable low cost periodontal disease diagnostic", (QMUL/AL05), June
2006-May 2008
£48,000 IP2IPO Ltd GRUB funding awarded to Degrasense for a project
entitled "Prototype sensor for periodontal disease monitoring" July
2008-June 2009
£12,000 Combined London Colleges University Challenge Partnerships (CLUC)
awarded to Degrasense for a project entitled "Disposable low cost
periodontal disease diagnostic", July 2008-June 2009
Details of the impact
The American Dental Association estimates that there are around 300
million periodontal examinations undertaken in the USA every year,
representing ca. 30% of the examinations undertaken world-wide. Based on
Watkinson and Krause's research, QMUL's spin-out company Degrasense
(established in January 2008) has developed a system with the likely
capability to accurately identify periods of active inflammation in
relation to periodontal disease that is inexpensive to mass produce. The
meter (Figure 1) is a point of care system that is designed to allow
rapid, in-house diagnosis of periodontal disease, precluding the need to
outsource assays to external laboratories. It has been estimated that the
consumables associated with this novel technology, will have a market
value of approximately 450m GBP per annum (Oraldent — see below). The
technology will improve the targeting of treatment to patients with active
periodontitis and ultimately improve clinical outcomes and enhance patient
experience.
The device developed by spin-out company Degrasense is shown in Figure 1.
Briefly, the meter works by samples of gingival crevicular fluid (GVC)
from the gums, being loaded onto the capillary sensor chip, which contains
electrodes coated with the QMUL patented technology. This sensor is then
loaded into the machine, where protelytic activity of the clinically
relevant proteases in the GVC is measured through changes in electrical
impedance.
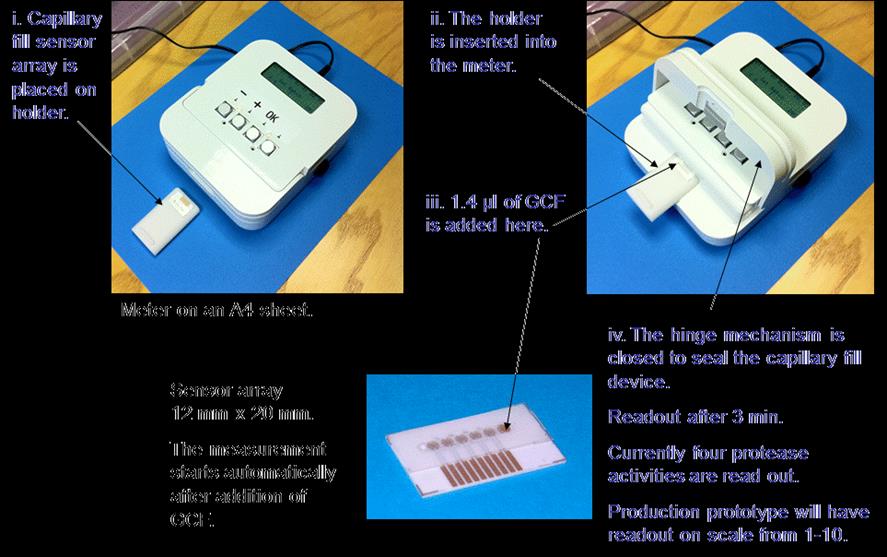 Figure 1: Meter used to detect protease activity in patients with periodontal disease
Figure 1: Meter used to detect protease activity in patients with periodontal disease
Companies and charities influenced by the research
To reach the point of a clinical trial [below, and 1 in section 5]
required collaboration with a number of industrial partners. Supported by
Technology Strategy Board funding, Watkinson coordinated the industrial
partners to develop the technology specifically for the clinical
diagnostics market. These partners are:
- Oraldent (Oraldent: www.oraldent.co.uk)
who assessed the size of the potential periodontal market to be 450m GBP
per annum in consumables (value of disposable capillary sensor chip).
- Industrial Design consultancy (IDC: www.idc.uk.com)
[2 in section 5] who designed the meter (see Figure 1)
- AND Technology Research (AND: http://andtr.com)
who developed the electronics in the meter [3 in section 5].
- Gwent Electronic Materials Ltd. (www.gwent.org/gem_index.html)
who manufactured the interdigitated electrodes used in the clinical
trial [4 in section 5].
- Barts Charity (www.bartscharity.org.uk)
who are currently funding Gwent Electronic Materials to further refine
the electrodes based on insights gained through the clinical trial.
Collectively, these industrial partners contributed over £340k to the
project.
Development of a new clinical diagnostic tool and its application
in a clinical trial
The efficacy of the Degrasense technology is currently being assessed via
a clinical trial involving 30 patients suffering from chronic
periodonitis. This is an important step on the road to delivery of this
diagnostic technology to market. The clinical trials are being undertaken
by staff within the School of Clinical Dentistry at the University of
Sheffield, in partnership with Sheffield Teaching Hospitals NHS Foundation
Trust. The aim of the trial is to assess whether the device can be used to
detect active periodontal disease.
The initial 12-month stage of the trial necessitated the appointment of a
dental hygienist to collect patient samples. The patients were recruited
from a number of sources within the partnering hospital, including the
undergraduate periodontology teaching clinic, staff hygienist clinics and
consultant clinics. All patients were selected by the clinician in charge
[1 in section 5] according to criteria that include; aged 18 or over; a
diagnosis of chronic periodontitis but otherwise healthy and; one healthy
gingival crevice, one deep bleeding and one deep non-bleeding periodontal
pocket.
Data from the first 12 months of the trial have revealed that the
interdigitated electrodes produced by Gwent Electronics need further
refinement. The company, funded by Barts Charity, are currently modifying
the electrodes to increase reliability. All patient samples from the first
stage of the trial have been retained for re-testing once these
refinements are in place.
Application of Degrasense technology in other clinical settings
Krause and Watkinson's technology has already attracted significant
interest and investment from multiple industrial partners and its use in a
clinical setting has been demonstrated through application in a clinical
trial. In addition to increasing the efficacy of diagnostic tools for
assessing periodontal disease, the technology has significant potential in
a number of other clinical applications where elevated levels of
proteolytic enzymes are linked to disease states. Investigations into
these applications are ongoing (and are sensitive at this stage) but
include: (i) home monitoring of haemophilia; (ii) clinical monitoring of
sepsis and trauma; (iii) home monitoring of multiple sclerosis and (iv)
the monitoring of arterial ageing and the role of proteinases in
hypertension. All of these areas are associated with very significant
patient numbers and cost to the NHS. The generic technology that Watkinson
and Krause have developed is capable of providing significant improvements
in the quality of patient monitoring and consequently also reduction in
treatment costs.
Sources to corroborate the impact
- Professor and NHS Consultant, The University of Sheffield and
Sheffield Teaching Hospitals NHS Foundation Trust: the clinician
currently undertaking the clinical trial.
- Programmes Director, AND Technology Research Ltd: this company
developed the meter being used in the clinical trial through the TSB
project.
- Project Manager, Industrial Design Consultancy: AND Technology
Research Ltd sub-contracted the meter design to Industrial Design
Consultancy Ltd.
- Technical Director, Gwent Electronic Materials: GEM Ltd manufactured
the interdigitated electrodes currently being used in the clinical trial
in Sheffield and are printing new electrodes coated with the degradable
polymer for the ongoing multiple sclerosis study.
- Director, QED Biosciences: QED provided an independent assessment of
the technology for the IP-Group.