1. Q Chip Ltd - Micro Technology for Injectable Therapeutics
Submitting Institution
Cardiff UniversityUnit of Assessment
General EngineeringSummary Impact Type
TechnologicalResearch Subject Area(s)
Chemical Sciences: Analytical Chemistry, Macromolecular and Materials Chemistry
Engineering: Interdisciplinary Engineering
Summary of the impact
Economic impact is claimed through the growth of the biopharmaceutical
spin-out company Q Chip Ltd. During the REF period, this has created 19
new jobs, £7.5M investment, a new Dutch subsidiary (Q Chip BV), and
staged-payment, six figure contract sales to four major international
pharmaceutical companies.
Q Chip has generated over £928K in contract sales from the pharmaceutical
industry from 2008-2012, with further sales of over £1M projected in
2013-14.
Originally established by Professor David Barrow in 2003 from his micro
technology research, Q Chip has developed new processes and miniaturised
equipment to encapsulate materials, including drugs, within uniform
polymeric microspheres as injectable therapeutics.
Underpinning research
Since 1995, Barrow has led the specialist research field of
microchip-based, multiphase-microfluidics, at Cardiff University, focused
on precision polymeric microsphere production. Specific research
undertaken by Barrow (Professor since 2000) and co-workers between
1998-2002 resulted in the development of a new manufacturing technology
that creates uniform microspheres within planar microfluidic ducts (Fig.
1). Two phases of grants from EPSRC (GR/M29634, July 1999, £105k;
GR/M73026, May 2000, £293k) and TSB (TI NCBT, 6/25 May 2002, £50k)
resulted in two patents, now assigned to Q Chip [3.1], [3.2]. The
co-workers were post-doctoral staff Dr. Nicola Harries and Dr. Kostas
Bouris, and PhD research associate Mr Tyrone Jones, all employed at
Cardiff University between 1998-2001.
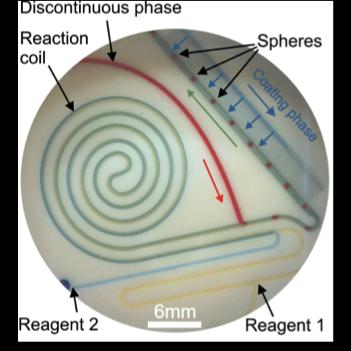
Figure 1: Microsphere production chip. Reagents 1 and 2
are precision delivered through flat capillaries, in which they are
incubated within a reaction coil. After this they are interfaced with an
immiscible discontinuous phase, thus causing the formation of droplet
microspheres, which are then coated.
The first phase focused on the method of generating ideal fluid mixing
conditions required to produce uniform fluidic droplets (shown in Fig. 2),
initially by modelling [3.3] and then by experiment [3.4]. This research
discovered and characterised the many inter-related microfluidic
geometries for the generation of segmented flow streams, and their
manipulation to engineer uniform microspheres. A key advantage of this
particle manufacturing technique is the rapidity, purity, and uniformity
of products, the low-shear aseptic environment (thus preventing damage to
living cells), and the ability to undertake precision, serial chemical
operations on individual microspheres. It had not been possible to achieve
these features using traditional manufacturing techniques. At that time,
only a few other scientists were investigating similar phenomena. Since
then, the area of multiphase microfluidics using planar chip-based
substrates has developed rapidly and is now heavily researched and is used
in diverse applications. Subsequent related research by Barrow and new
co-workers has continued, with new inventions such as the liquid phase
separator [3.5].
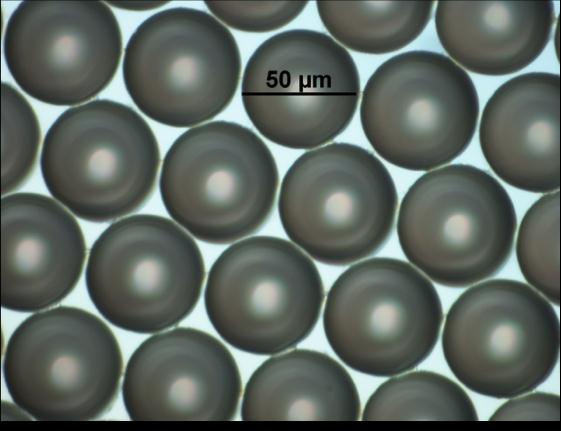
Figure 2: Polymeric microspheres, with a highly uniform
diameter of 50 microns.
The second parallel phase of research identified combined manufacturing
mechanisms and materials required to create the microfluidic chips for
fluidic processing [3.1]. The mechanism involved novel, deep
plasma-etching of microfluidic structures on bioinert, solvent-resistant,
fluoropolymer substrates (shown in Fig. 3). This enabled highly-regular
microparticle formation, without the undesired use of surfactants, some of
which are toxic. This novel wafer-processing method favoured
solvent-resistant polymers and is compatible with processing equipment
already used in industry for silicon (like silicon-based,
high-aspect-ratio structures; for example, airbag sensors and
accelerometers).
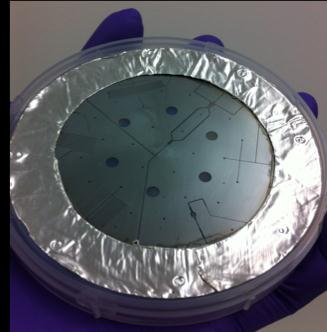
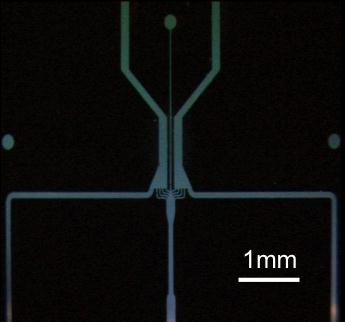
Figure 3: A four inch PTFE wafer, with a metal mask,
showing deep-etched microfluidic ducts (top). Fine detail of the
microfluidic duct system on this wafer is also shown (bottom).
Pilot manufacturing studies, initiated by Barrow in 2001, established a
desk-top manufacturing microPlant for the production of microspheres
[3.6]. This has been employed for payload delivery in multiple industries,
such as biopharmaceuticals, neutroceuticals, cosmetics and smart paints.
An independent commercialisation report from Biolauncher Ltd. (contact
Rowan Gardener, Biolauncher Ltd., www.Biolauncher.com)
proposed that significant revenues would be obtained by focusing product
development on high-value, low-volume markets (including pharmaceuticals
delivery) and this strategy has since been followed.
With initial Welsh Assembly Government support (Q Chip exploitation roadmap, Education and Learning Wales Ref. 112; 01/08/02), and working with Entegris
and Victrex corporations, Barrow produced a platform [3.6]
for bespoke microsphere production to demonstrate the technology to
potential investors and supply chain partners.
The market potential for the Cardiff University research was supported by
Barrow, as inventor, patent author, pre-seed development leader, Q Chip
co-founder, and Q Chip Chief Scientific Officer. He exemplified the
initial prospects through exploratory work from 1999-2000, for Unilever
UK, by demonstrating pectin fibre production for hair-care products (Hugh.Clare@liv.ac.uk,
at Liverpool University, previously at Unilever).
Q Chip Ltd. was founded in 2003 by Prof. Barrow, Mr Mark Barry and Dr Jo
Daniels (Dr Nick Bourne, Cardiff University, Bourne@cf.ac.uk,
and documents kept therein). The company secured first round funding from
the E-Synergy investor syndicate (lead investor John Moulton), with a
corresponding second closing IP-equity swap with Cardiff University.
References to the research
3.2 Barrow D.A., Harries N., Jones T.G. and Bouris K.
(2004) Microfluidic device and methods for construction and application, Patent
Numbers: UK GB2395196B, Worldwide CA2545205, UK0226691.4, filing
date 11/11/2002.
http://www.google.com/patents/US7802591
3.3 Barrow D.A., Harries N., Burns J.and Ramshaw C. (2003) A
numerical model for segmented flow in a microreactor, International
Journal of Heat and Mass Transfer, Vol. 46 No. 17 pp. 3313-3322, http://dx.doi.org/10.1016/S0017-9310(03)00120-0
3.4 Ahmed B., Barrow D.A. and Wirth T. (2006) Enhancement
of reaction rates by segmented fluid flow in capillary scale reactors, Advances
in Synthesis and Catalysis, Vol. 348 No. 9 pp. 1043-1050, ISSN
1615-4150 10.1002/adsc.200505480
3.5 Castell O.K., Allender C.J. and Barrow D.A. (2009)
Liquid-liquid phase separation: characterisation of a novel device capable
of separating particle carrying multiphase flows, Lab Chip, Vol. 9
pp. 388-96, 10.1039/b806946h
(this is output Barrow 3)
3.6 Velten T., Ruf H.H., Barrow D.A., Aspragathos N., Lazarou P.,
Jung E., Malek C.K., Richter M., Kruckow J. and Wackerle M. (2005)
Packaging of bio-MEMS: strategies, technologies and applications, IEEE
Transactions on Integration and Packaging, Vol. 28 No. 4 pp.
533-546,10.1109/TADVP.2005.858427
Details of the impact
Impact summary: During the REF period, employee numbers at Q Chip
increased from 10 to 29 (up 180%), there were 5 patent filings and there
was £7.5M of new investment (75% of the total company investment) over 6
funding rounds [5.1],[5.2]. Access to new miniaturised industrial
bioprocessing equipment and new drug delivery techniques (currently under
trials) were sold to top pharmaceutical companies for improved quality of
life through cancer treatment. Revenues from four, staged payment,
six-figure value contracts [5.1],[5.2] have risen from £17k in 2010, £297k
in 2011 to £578k in 2012.
Company growth: During the REF period Q Chip has developed a
bio-encapsulation and drug delivery platform that enables companies to
extend product life cycles and deliver complex bio-therapeutics [5.3]. It
currently manufactures drug-loaded microspheres, mainly for pharmaceutical
industries. Q Chip aims to improve patient compliance and experience, and
improve therapeutic performance through the development of long-acting,
injectable therapeutics using its proprietary platform, Q-SpheraTM [5.3].
This breakthrough microsphere manufacturing and formulation system is
compatible with small molecules, peptides and complex biologicals.
With its commercial client partners, and with guidance from its
scientific advisory board (on which Barrow sits), Q Chip is developing
point-of-care clinical data with three different molecules encapsulated
within drug-eluting, polymeric microspheres. Q Chip is licensing the
microsphere production and sale via these client partners who, through
their own existing global marketplace presence, are taking the products to
market. Q Chip has five injectable, encapsulated drug formulations in its
therapeutics pipeline, including Q-Goserelin and Q-Leuprolide for the
treatment of breast and prostate cancer, Q-Insulin for diabetes therapy,
Q-Octreotide for acromegaly, and a new formulation of monoclonal
antibodies for a confidential application [5.1]. All have substantial
existing markets. For instance, Leuprolide and Octreotide products
achieved annual sales of around $1800M and $1200M in 2010; this is out of
a total global market for injectable therapeutics of $49B. During 2011, Q
Chip signed four separate multi-year R&D deals with international
pharmaceutical companies [5.1]. Two of these contracts are with those in
the top five global pharmaceutical industries. Q Chip has developed a
contractual relationship with leading biotechnology/pharmaceutical
company, ARTES Biotechnology GmbH [5.4], for the sustained release of
therapeutic protein interferon alpha 2 (using microspheres) to treat
chronic hepatitis B and C. A third contract is with a UK pharmaceutical
company, a fourth with a mid-sized European pharmaceutical company [5.1]
(contractually, other company names cannot be disclosed).
Further technical development: During the REF period Barrow has
continued to be instrumental in determining the on-going evolution of the
technology developed through the underlying research into Q Chip's
expanding range of products. He has also supported the company as chair
and co-chair of Q Chip's Scientific Advisory Board. His involvement has
enabled access to his TSB funded metaFAB facility for HPLC-MS and
femtosecond laser micromachining, and has enabled additional two-way
development contracts between the University (Professor Steve Dunnet, Dr.
Peter Kille) and Q Chip. The latter contracts developed cell-encapsulation
capabilities for the company, using its core microsphere production
technology.
Since Q Chip's inception, there has been significant process development
and intellectual property protection, through five patents, based on its
collaborative research programme. Microspheres are produced using a hybrid
of the original IP with new geometries and sub-systems. This happens at a
greater rate, with less solvent waste, using automated, industry-ready
bespoke equipment, also manufactured by Q Chip. The Q-Sphera manufacturing
process leads to environmental improvements and is now much `greener' than
current methods because (i) it operates without Class II
halogenated/hazardous solvents (e.g. dichloromethane/chloroform), (ii) it
uses >80% aqueous solvents (so waste may be recycled to remove Class
III solvents such as DMSO, alcohols), (iii) it is far more efficient than
current methods since there is no need for size-fractionation (no wastage
of intermediary product since there is none), and (iv) the unique, highly
miniaturised, production-on-demand processing equipment (manufactured by Q
Chip) sits on a desk, rather than occupying a building [5.1]. In 2011,
through a £3.6M external investor round, the Q Chip BV European
manufacturing subsidiary was founded as a cGMP sterile pharmaceutical
production facility at Geleen, near Maastricht, with initially three FTE
staff [5.5]. In 2012, the company was awarded a TSB grant of £250k towards
the cost of developing its innovative, scaled-up, sustained-release
pharmaceutical manufacturing process and production equipment [5.1].
Company investment: To provide context to the company investments
during the REF period, it is valuable to know that before the
REF period, the business secured first round funding from the
E-Synergy investor syndicate in 2004 (lead investor John Moulton), with a
corresponding second closing IP-equity swap with Cardiff University, which
now holds about 3% equity. Since its incorporation in 2003, Q Chip has
attracted investment of £10.4M over 12 funding rounds from angel and
institutional investors, has over 57 shareholders, with 29 FTE staff who
have contributed to employment in Wales [5.6].
During 2011-12, existing shareholders, including Disruptive Capital
Finance and Finance Wales, were joined by venture capitalist Jon Moulton
(founder of Better Capital) [5.7] and industrial entrepreneur Sir Harry
Solomon, in two £5.6M Series C financing rounds led by Limburg Ventures
and Nedermaas Hightech Ventures [5.7],[5.8]. Q Chip uses these funds to
increase production by scaling-up manufacturing development of
encapsulated drugs for prostate cancer and acromegaly.
Finally, in 2011-12 EPSRC undertook a healthcare economic IMPACT Study on
Q Chip, since its origins were based on EPSRC-funded research (IMPACT
summarised in [5.9] and [5.10]).
Sources to corroborate the impact
5.1 Confirmation of employment generated, investment and contracts
achieved and that Q-Chip developed 5 injectable microsphere formulations
on company contracts from Chief Operating Officer Q Chip Ltd.
5.2 Confirmation of turnover from audited (Ernst & Young) annual
company reports (Companies House Company No. 04929486).
5.3 Confirmation of Q Chip's specialist drug delivery mechanisms and
their benefits at
http://www.q-chip.com/downloads/0723_Inside_Technology_article.pdf.
5.4 Confirmation of a contractual relationship with leading
biotechnology/pharmaceutical company, ARTES Biotechnology GmbH http://www.artes-biotechnology.com/news.jsp.
Article on 29.04.2010.
5.5 Confirmation of investment and establishment of Dutch manufacturing
subsidiary http://www.growthbusiness.co.uk/news-and-market-deals/fundraising-deals/1625353/jon-
moulton-backs-q-chips-series-c-financing-round.thtml.
5.6 Confirmation from the (then) Welsh Assembly Government Minister, Mr
Andrew Davies, of economic (including employment) impact of Q Chip.
5.7 Confirmation of £3.6M investment raised http://www.walesonline.co.uk/business-in-
wales/business-news/2011/06/14/q-chip-raises-3-6m-more-to-expand-91466-28871341/
5.8 Confirmation of £2M funding from external investors in 2012 http://www.insidermedia.com/insider/wales/81880-2m-funding-q-chip/index.html.
5.9 Confirmation of original EPSRC funding from: "EPSRC Pioneering
Healthcare Technologies for the UK Life Science Sector", [No 3 on page 5].
One of four IMPACT case studies selected for this EPSRC publication.
5.10 Confirmation of economic impact from `EPSRC Research Performance and
Economic Impact Report 2011/12' [Page 13 ] at: (paste hyperlink into
google address bar on macs): http://www.epsrc.ac.uk/SiteCollectionDocuments/Publications/corporate/ResearchPerforman
ceAndEconomicImpactReport2011-12.pdf.