Mercury Capture Technology for the Global Petroleum Industry
Submitting Institution
Queen's University BelfastUnit of Assessment
ChemistrySummary Impact Type
TechnologicalResearch Subject Area(s)
Chemical Sciences: Organic Chemistry, Physical Chemistry (incl. Structural), Other Chemical Sciences
Summary of the impact
Queen's University's Ionic Liquids Laboratory (QUILL) has developed an
ionic liquid technology for removing mercury, a toxic, corrosive
contaminant naturally present in hydrocarbon reserves, with the national
oil and gas company Petroliam Nasional Berhad (PETRONAS).The technology
has been successfully installed in 1-and 15-ton units in two PETRONAS gas
processing plants in Malaysia. The process, marketed as HycaPure Hg™,
captures all mercury species present in natural gas and has up to 3 times
higher capacity than competing state-of-the-art commercial alternatives.
This technology represents a significant improvement towards ensuring the
health and safety of workers, process plant and the environment.
Underpinning research
QUILL was the first research centre to focus on the development of ionic
liquids (liquid salts) and is now recognised as the world-leader in new
applications, notably in green chemistry. We drew on our expertise to
design an entirely new ionic liquid based approach to remove mercury from
natural gas supplies, a feat that has much broader applications. The
underpinning research has been performed since the formation of QUILL in
1999 to now. Over this period of time a significant understanding of the
controlling features of ionic liquids was determined from a fundamental
perspective. This included the phase behaviour of ionic liquids and the
effect of anion and cation structure as well as new synthetic methods for
the production of low cost ionic liquids and the effect of impurities
(halide and water, for example) on physicochemical properties of the
materials (references 1-5 in section 3). Due to the large number
of ionic liquid systems possible, predictive methods for the determination
of both chemical interactions and physical property determination were
also formulated (references 4 and 5 in section 3). Using this
understanding and a knowledge of the requirements of the challenge —
notably the need to extract inorganic, organic, and elemental forms of
mercury from natural gas streams on an industrial scale, and the need for
rapid capture kinetics in order to protect down-stream facilities from
spikes in mercury content, Seddon, Nockemann and Holbrey led the team
which targeted the design, synthesis and testing of ionic liquid materials
at laboratory scale. Following the initial screening, the Queen's team
successfully incorporated the active ionic liquids into porous solids
without leaching. Finally, the composition of the solid-supported ionic
liquid (SSIL) was optimised as a direct retrofit to existing mercury
scrubbers with no added investment required (reference 6 in section 3).
In partnership with PETRONAS, the optimised SSIL was scaled-up and
validated at pilot scale and subsequently scaled up to 15 tons of
adsorbent providing the first commercial charge to treat natural gas at an
on shore PETRONAS gas processing plant.
References to the research
* signify the references which best indicate the quality of the
underpinning research
Key references from the investigators, demonstrating the strength and
depth of fundamental research underpinning the design, understanding and
application of ionic liquid materials.
1. Influence of chloride, water, and organic solvents on the physical
properties of ionic liquids, KR Seddon, A Stark, MJ Torres, Pure Appl.
Chem., 2000, 72, 2275, DOI: 10.1351/pac200072122275. This is a key paper
which demonstrated to the ionic liquid research community the importance
of understanding and maintaining knowledge of the purity of ionic liquid
systems.
2. The phase behaviour of 1-alkyl-3-methylimidazolium tetrafluoroborates;
ionic liquids and ionic liquid crystals, JD Holbrey, KR Seddon, J. Chem.
Soc., Dalton Trans., 1999, 2133, DOI: 10.1039/a902818h. This fundamental
study demonstrates the effects of systematic structural changes on the
thermophysical properties of ionic liquid systems.
3. Efficient, halide free synthesis of new, low cost ionic liquids:
1,3-dialkylimidazolium salts containing methyl-and ethyl-sulfate anions,
JD Holbrey, WM Reichert, RP Swatloski, GA Broker, WR Pitner, KR Seddon, RD
Rogers, Green Chem., 2002, 4, 407, DOI: 10.1039/b204469b. New ionic
liquids were described prepared using simple, clean and halide-free
syntheses.
*4. Desulfurisation of oils using ionic liquids: selection of cationic
and anionic components to enhance extraction efficiency, JD Holbrey, I
Lopez-Martin, G Rothenberg, KR Seddon, G Silvero, X Zheng, Green Chem.,
2008, 10, 87, DOI: 10.1039/b710651c. The paper applied analysis of
structure-activity relationships to determine the `best' cations to use in
the design of ionic liquids for desulfurisation of diesel fuel.
*5. Small angle neutron diffraction from 1,3-dimethylimidazolium
chloride, C. Hardacre, J. D. Holbrey, S. E. J. McMath, D. T. Bowron, and
A. K. Soper, J. Chem. Phys., 2003, 118, 272, DOI: 10.1063/1.1523917. This
paper was the first to demonstrate the use of neutron scattering (using
STFC large-scale facilities at ISIS) to directly visualise ionic liquid
structure. This allows us to uniquely link bulk properties of ionic liquid
systems with their behaviour at the atomic level.
The materials that form the basis of the HycaPure™ technology are
proprietary knowledge and form a suite of 4 patents the most relevant
being:
*6. Process for removing metals from hydrocarbons, M. Abai, M. Atkins, K.
Y. Cheun, J. D. Holbrey, P. Nockemann, K. R. Seddon, G. Srinivasan, Y.
Zou, World Patent Application PCT/WO 2012/046057 A2
Details of the impact
QUB and PETRONAS in a unique partnership have developed a novel mercury
removal technology HycaPure Hg™ based on ionic liquids which offers
advantaged performance and flexibility to treat a full range mercury types
and gas composition (Figure 1). The first full-scale HycaPure Hg™ units
containing 1 and 15 tons of absorbent were installed at a PETRONAS gas
processing plant in Malaysia in Oct 2011 and have been successfully
producing ethane for PETCHEM and sales-quality gas since commissioning
(references 1-4 in section 5). The mercury content has been
consistently below the legal safe limits, with no excursions. The new
technology has a number of benefits over the competing commercial systems,
e.g. the kinetics of mercury absorption are 10-20 times faster, allowing
the operating plant to cope with large spikes of mercury with no risk to
the downstream plant, and the mercury absorption capacities of the new
materials are 2-3 times better on a volume/volume (reactor) basis. The new
system is a direct retrofit to commercial plants and installs without any
additional costs or modification to procedures (see PETRONAS' Technology
Products and Technical Solutions literature).
A typical medium-sized mercury removal unit contains around 15 tons of
material, and costs in the order of US$180,000 per fill. By comparison,
the increased absorption capacity of HycaPure Hg™ materials represents a
potential cost saving of over 20% per vessel, and with a market size
running into hundreds of thousands of tons, this new technology is not
only efficient but highly competitive.
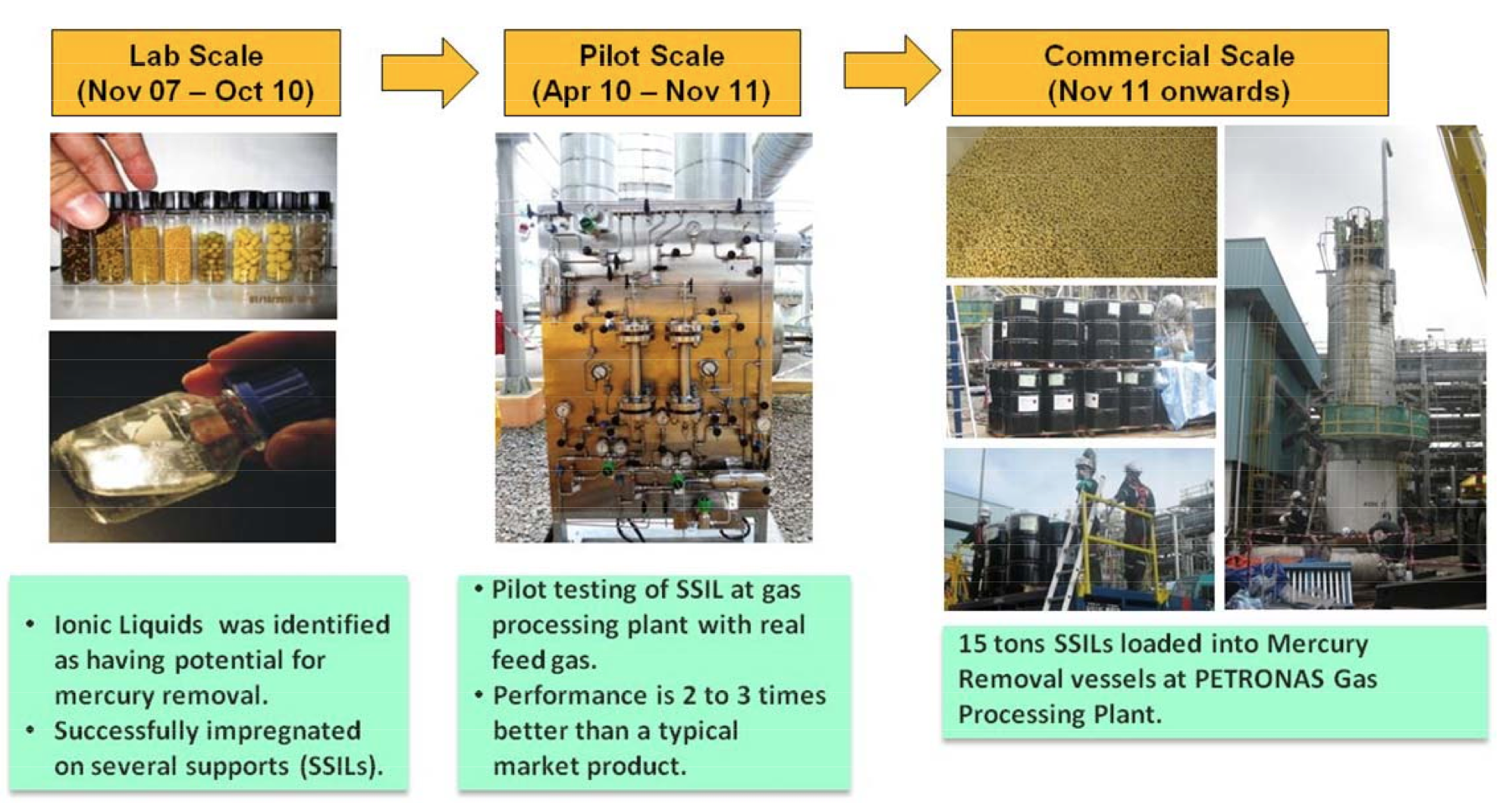 Figure 1 Development of the mercury capture technology
for natural gas clean up in conjunction with PETRONAS
Figure 1 Development of the mercury capture technology
for natural gas clean up in conjunction with PETRONAS
The potential market for our SSIL technology in the hydrocarbon industry
is huge: MRUs are required in almost every gas and oil terminal and
refinery/petchem complexes in addition to the produced waters from
drilling platforms. Mercury contamination ranges from 0.02 micrograms per
cubic metre in the Gulf of Mexico to more than 100 in Europe, South
America, Gulf of Thailand, Malaysia and Indonesia. In extreme cases, such
as in North Germany, levels can reach 5000 micrograms per cubic metre.
Even 1 ppm of mercury contamination has significant cumulative effects if
we consider a typical plant processes 2000 tons every day. In addition to
its well-documented health and environment effects, mercury also damages
industrial facilities through corrosion, such as embrittlement of
aluminium heat exchangers with catastrophic consequences. Hg is also a
strong catalyst poison for downstream units.
The current technologies used to remove mercury are chemically-modified
activated carbons (with sulphur for gas treatment, and potassium iodide
for liquid hydrocarbon treatment) and more expensive technologies, such as
silver-impregnated molecular sieves and mixed metal sulphide/oxide
scrubbers. But there are issues with these technologies when it comes to
efficiency, the removal of all types of mercury species, robustness when
other contaminants are present in the feed and the ability to deal with
fluctuating mercury levels. The HycaPure Hg™ is proving robust and durable
in operation. The technology is being extended to other gas treatment
facilties in PETRONAS facilities and licensing/manufacturing partners are
planning to launch the product globally in 2014. .
The (anticipated) greater life-time of HycaPure Hg™ scrubbers will lead
to lower total volumes of hazardous mercury-contaminated waste for
post-unit remediation and reduced frequency of scheduled scrubber
replacement, along with reduced occupational exposure to mercury. The
issue of mercury exposure was recently recognised by governments in a
global, legally-binding treaty to prevent emissions and releases, the
Minamata Convention on Mercury.
The impact of this work was recently recognised in the Government's Great
British Innovation Vote in March 2013 (reference 5 in section 5).
Sources to corroborate the impact
- The contribution of QUB, through the partnership with PETRONAS, is
acknowledged in a letter of corroboration from the Head of Technology
Management Department of Technology & Engineering Division,
PETRONAS.
- The PETRONAS Laboratory at the QUILL (Queen's University Ionic Liquids
Laboratories) Research Centre was officially opened by The Northern
Ireland Assembly Minister for Employment and Learning who recognised it
as "an exemplar of industry and academic collaboration, not just for
Northern Ireland but for the United Kingdom as a whole."
(http://www.northernireland.gov.uk/index/media-centre/newsdepartments/news-del/news-del-april-2008/news-del-170408-minister-opens-new.htm
and Petronas 2009 Annual Financial Statement, page 68 (www.petronas.com.my/investor-relations/Documents/annual-report/AnnualReport_FinancialStatement_2009.pdf)"
- 2011 Annual Financial Statement (www.petronas.com.my/investor-relations/Documents/annual-report/AnnualReport_FinancialStatement_2011.pdf)
Notes the collaboration with QUB and the first commercial commission of
HycaPure Hg™ at PETRONAS Gas Bhd (PGB).
- Strategic presentations describing the technology at the International
Gas Union Research Conference (19-21 Oct 2011, Seoul, Korea; http://www.igrc2011.com/programme),
EUCHEM 2012 (5-10 Aug 2012, Celtic Manor, Wales; http://www.euchem2012.org/index.php/scientific-programme)
and Green Solvents (7-10th October 2012, Boppard, Germany;
http://events.dechema.de/events/en/gsfs2012.html).
- Listed in the Government's Great British Innovation Vote, March 2013
http://www.topbritishinnovations.org/FutureInnovations/IonicLiquid.aspx