HLA Alleles as Genetic Predictors for Drug-Induced Hypersensitivity Reactions
Submitting Institutions
University of Liverpool,
Liverpool School of Tropical MedicineUnit of Assessment
Clinical MedicineSummary Impact Type
TechnologicalResearch Subject Area(s)
Medical and Health Sciences: Immunology
Summary of the impact
The University of Liverpool (UoL) research has had health impact on
immune-mediated drug hypersensitivity reactions, which can be severe and
life-threatening. It has shown that predisposition to hypersensitivity
reactions caused by abacavir, nevirapine, carbamazepine and flucloxacillin
is due to specific HLA genes on chromosome 6. This has led to changes in
the drug label and guidelines for abacavir, increased HLA-B*57:01
gene testing in the NHS through a University spin-out company, and a
reduction in the incidence of hypersensitivity from 7% to <1%. The more
recent demonstration of HLA-A*31:01 and predisposition to
carbamazepine hypersensitivity, has led to drug label changes for
carbamazepine.
Underpinning research
The research described was all undertaken at the UoL after 1993 and led
by Professors Munir Pirmohamed and Kevin Park and Dr Ana Alfirevic (Snr
Lecturer).
Adverse drug reactions (ADRs) account for 6.5% of all hospital admissions
(Pirmohamed et al, BMJ. 2004; 329:15-9). Immune-mediated ADRs
(also called hypersensitivity reactions) are not predictable from the
pharmacology of the drug and can lead to fatalities. The reactions are
mediated by antigen-specific T cells interacting with HLA-restricted
antigen presenting cells, providing a functional rationale to the
potential role of the major histocompatibility complex (MHC), and the HLA
alleles contained therein, as markers of susceptibility. The research in
this area began in 2000, with our first publication in 2001 showing the
importance of genes within the MHC, including HLA alleles, in
carbamazepine-induced hypersensitivity [1].
Abacavir is an antiretroviral that can cause life threatening
hypersensitivity in 5-7% of patients, with rechallenge leading to severe
and fatal reactions. Work undertaken in Australia in 2002 showed that HLA-B*57:01
predisposed to abacavir hypersensitivity. UoL research undertaken between
2002 and 2004, which included the identification and recruitment of
abacavir hypersensitivity patients and controls [2], showed that not only
was HLA-B*57:01 associated with the reaction, but in addition, it
was the first study to demonstrate the cost effectiveness of pre-
treatment testing for HLA-B*57:01 in preventing hypersensitivity.
Carbamazepine, an anticonvulsant, can cause a wide range of
hypersensitivity reactions including dangerous or even fatal skin
reactions. In 2004, a Taiwanese group reported that all Han Chinese
patients who experienced carbamazepine-induced Stevens-Johnson syndrome
(SJS) were positive for HLA-B*15:02. UoL research showed that this
allele was not important in patients of European ancestry because of its
low prevalence [3], and it did not predispose to another type of
hypersensitivity reaction called DRESS (drug reaction with eosinophilia
and systemic symptoms). A recent UoL systematic review has shown that HLA-B*15:02
is important in Chinese, Thai, Malay and Indian populations, but not in
Caucasians or Japanese [4].
In March 2011, UoL research showed that HLA-A*31:01 is associated
with CBZ-hypersensitivity reactions in Caucasians [5]. HLA-A*31:01,
unlike HLA-B*15:02, is associated with all forms of
hypersensitivity reactions to CBZ (including maculopapular eruptions,
hypersensitivity syndrome and SJS). The same finding has also been
demonstrated in Japanese patients. The finding is particularly important
given the wider worldwide distribution of HLA-A*31:01
compared with HLA- B*15:02 which is found predominantly in South
East Asia.
The research has investigated other hypersensitivity reactions:
e.g. it has shown that HLA- B*57:01 is a predisposing factor for
flucloxacillin hepatitis (Daly et al; Nat Genet 2009;41:816-9) and HLA-C*04:01
is a predisposing factor nevirapine-induced SJS in Malawians [6].
References to the research
1. Pirmohamed M, Lin K, Chadwick D, Park BK
(2001). TNF-α promoter region gene polymorphisms in
carbamazepine-hypersensitive patients. Neurology, 56, 890-896 (PMID:
11294926) Citations: 96 Impact Factor: 8.249
2. Hughes DA, Vilar FJ, Ward CC, Alfirevic A,
Park BK, Pirmohamed M. (2004). Cost- effectiveness analysis
of HLA B*5701 genotyping in preventing abacavir hypersensitivity.
Pharmacogenetics, 14, 335-342 (PMID: 15247625) Citations: 147 Impact
Factor: 3.608
3. Alfirevic A, Jorgensen AL, Williamson PR, Chadwick DW,
Park BK, Pirmohamed M. (2006). HLA-B locus in Caucasian
patients with carbamazepine hypersensitivity. Pharmacogenomics, 7, 813-818
(DOI: 10.2217/14622416.7.6.813) Citations: 122 Impact Factor: 3.857
4. Yip VL, Marson AG, Jorgensen AL, Pirmohamed
M, Alfirevic A. (2012). HLA genotype and
carbamazepine-induced cutaneous adverse drug reactions: a systematic
review. Clin Pharmacol Ther, 92, 757-765 (DOI: 10.1038/clpt.2012.189)
Citations: 5 Impact Factor: 6.846
5. McCormack M, Alfirevic A, Bourgeois S, Farrell JJ,
Kasperaviciute D, Carrington M, Sills GJ, Marson T, Jia X, De
Bakker PI, Chinthapalli K, Molokhia M, Johnson MR, O'connor GD, Chaila E,
Alhusaini S, Shianna KV, Radtke RA, Heinzen EL, Walley N, Pandolfo M,
Pichler W, Park BK, Depondt C, Sisodiya SM, Goldstein DB, Deloukas
P, Delanty N, Cavalleri GL, Pirmohamed M. (2011). HLA-A*3101 and
carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J
Med, 364, 1134-1143 (DOI: 10.1056/NEJMoa1013297) Citations: 156 Impact
Factor: 51.658
6. Carr DF, Chaponda M, Jorgensen AL, Castro EC, Van Oosterhout
JJ, Khoo SH, Lalloo DG, Heyderman RS, Alfirevic A,
Pirmohamed M. (2013). Association of human leukocyte antigen
alleles and nevirapine hypersensitivity in a Malawian HIV-infected
population. Clin Infect Dis, 56, 1330-1339 (DOI: 10.1093/cid/cit021)
Citations: 0 Impact Factor: 9.374
Key research grants
2007-2013. The Department of Health. Department of Health Chair
in Pharmacogenetics, £3.3m (£33,000 supplement; 2009), PI M Pirmohamed,
CoI BK Park
2008-2013. Wolfson Foundation. Wolfson Centre for Personalised
Medicines, £2m, PI M Pirmohamed
2008-2013. MRC. MRC Centre for Drug Safety Sciences, £3.7m,
Director BK Park, Deputy Director M Pirmohamed
2006-2009. The Wellcome Trust. The Pharmacology of Nevirapine in
Malawian Patients: Implications for Dosing and Understanding of
Hypersensitivity Reactions, £438,229. (Clinical Training Fellowship for
Chaponda, Pirmohamed primary supervisor)
2010-2013. EU FP7 Initial Training Network. Priorities and
Standards in Pharmacogenomic Research: Opportunities for a Safer and More
Efficient Pharmacotherapy, €3.2m (£936,049 to Liverpool), PI M Pirmohamed
2010-2013. Serious Adverse Event Consortium. Development of the
International Consortium on Drug Hypersensitivity, £300k, PI M
Pirmohamed
2010-2016. MRC (and Industry partners including GSK, AZ, ICON). North
West England MRC Fellowships in Clinical Pharmacology and Therapeutics,
£3,012,100, Programme Leader M Pirmohamed
Details of the impact
Research at the University of Liverpool has collectively improved
understanding of the genetic basis of drug-induced hypersensitivity and
provided "proof of principle" for the clinical use of genetic testing
before prescribing drug therapy. UoL research has contributed to changes
in the prescribing information for two widely used drugs,
carbamazepine and abacavir. The prescribing information has been revised
to include information on the genetic predisposing factors to
hypersensitivity reactions caused by these two drugs; clinical
guidelines have also been changed for abacavir to reflect the need
for genotyping prior to drug administration. The research has impacted on
the clinical care and lives of patients with chronic diseases such as
epilepsy, bipolar disorder, neuropathic pain and AIDS. Therapy has been
optimised by individualising drug prescription to a patient based on key
characteristics such as genetic factors. This reduces the risk of severe
and potentially fatal ADRs.
Abacavir hypersensitivity
The study showed that it would be cost-effective to undertake
pre-treatment screening for HLA- B*57:01 to predict susceptibility
to abacavir hypersensitivity [2]. This has led to the following impacts.
- Clinical guidelines from societies, e.g. the British HIV Association,
were changed in 2008 to recommend testing for HLA-B*57:01 before
the use of abacavir [7]. This is now well established in NHS practice.
- The drug labels (i.e. prescribing information) were changed in the US,
EU and Australia after 2008 to recommend the use of HLA-B*57:01
genotyping prior to the use of abacavir [8-10].
- The evidence from the UoL study was used by the NHS to implement the
use of the HLA-B*57:01 testing in HIV clinics from 2006. This has led to
a marked reduction of the incidence of abacavir hypersensitivity (from
7.8% to 2% [11]). It is now rare to see any cases of abacavir
hypersensitivity in clinical practice to the benefit of patients — this
has been demonstrated in a publication from the Chelsea and Westminster
HIV clinic which showed that the incidence of hypersensitivity had gone
down from 7.8% to 2% [11]
- The number of HLA-B*57:01 genetic tests undertaken in the NHS rose
sharply after 2006, and at the same time the use of abacavir also
increased (see figure), and continues to the present day.
- Most of the HLA-B*57:01 testing was provided by Delphic
Diagnostics, a University of Liverpool spin-out company (co-founders were
Prof Khoo and Back, Dept of Pharmacology, UoL). Delphic was bought out by
Lab21 in 2009. Total number of tests in 2007-08 and 2008-09 were 3,493
(revenue £186,850) and 1,908 (£100,630) respectively.
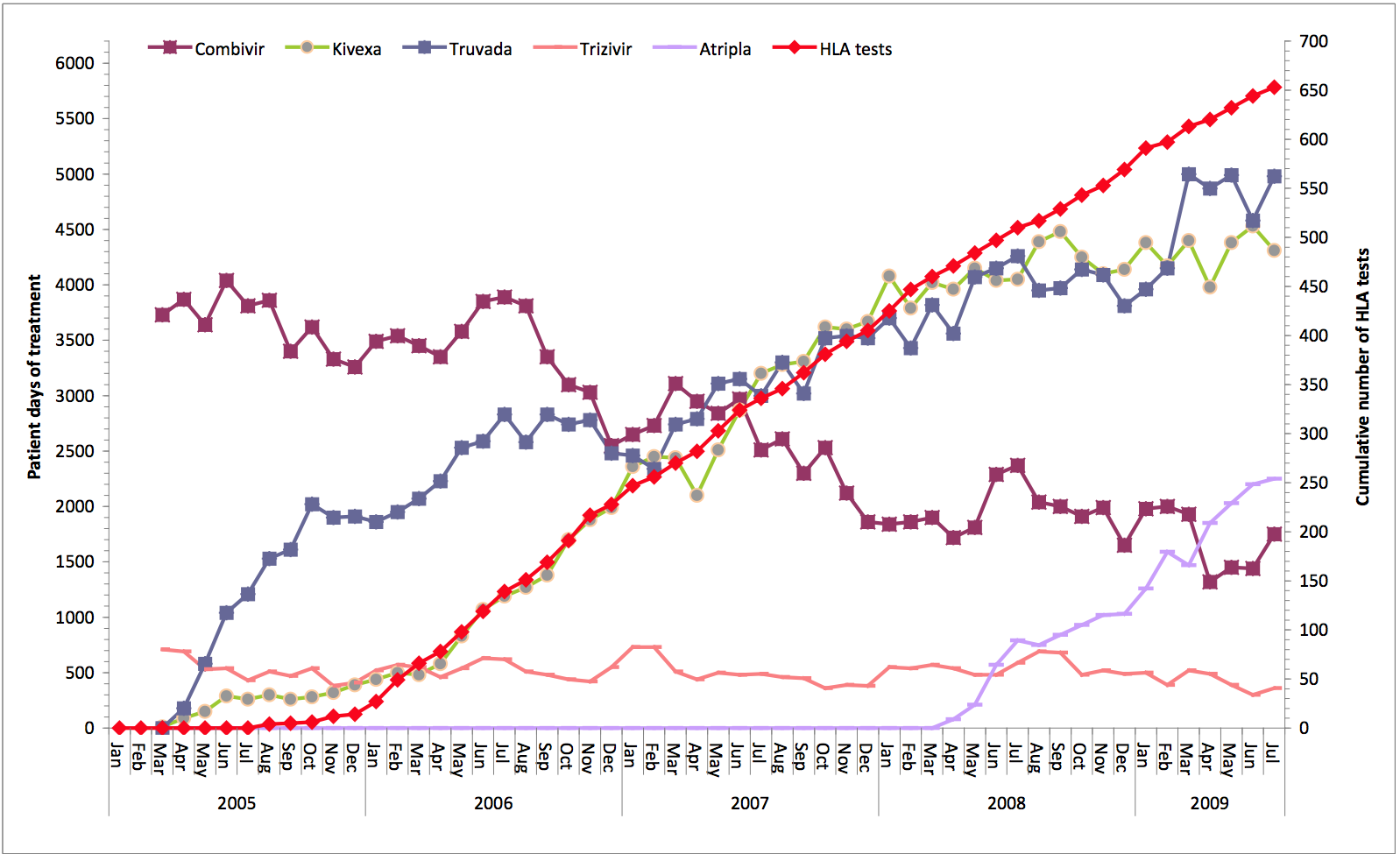 Figure: The increase in HLA testing and at the same time, the rise in the use of abacavir (kivexa)
Figure: The increase in HLA testing and at the same time, the rise in the use of abacavir (kivexa)
The importance of this work is confirmed by a case study by The Academy
of Medical Sciences in 2012 [12]. Pre-prescription genotyping for HLA-B*57:01
is now used in most countries and has also been shown to improve clinical
outcomes (i.e. reduce hypersensitivity) in Australia and France, and has
been shown to be cost-effective in several countries including the US and
Germany.
Carbamazepine hypersensitivity
UoL research showed susceptibility to carbamazepine hypersensitivity
reactions in Caucasian patients was localised to the MHC, but that the
risk factor in Caucasians was distinct to that demonstrated in Chinese
patients [3]. Thus, in Caucasians, HLA-B*1502, is not a risk
factor for SJS/TEN and for hypersensitivity syndrome, two distinct
phenotypes associated with carbamazepine treatment. This publication was
utilised by the FDA, who in December 2007, recommended that patients of
Asian ancestry, but not Caucasians, should be tested for HLA-B*1502
prior to the start of carbamazepine therapy [13]. The drug label was also
changed in the EU in 2008, again recommending the use of testing in Asian
patients, but not in Caucasians [14].
The continuing work of UoL in this area has recently identified HLA-A*31:01
as a genetic risk factor for carbamazepine-induced hypersensitivity
reactions in Caucasians [5]. The prescribing information for carbamazepine
has been changed in Japan, in the EU and in the US since 2011 making
prescribers aware of the association between this allele and carbamazepine
hypersensitivity in Caucasians [15].
In 2010, the UoL became the global co-ordinating centre for the
International Consortium on Drug Hypersensitivity (ITCH), sponsored by the
International Serious Adverse Events Consortium (iSAEC) (http://www.saeconsortium.org/).
We have now recruited 1500 patients with hypersensitivity reactions from
12 international centres, and 50 UK centres.
In 2013, we were awarded an i4i grant from the NIHR in collaboration with
MC Diagnostics to develop a HLA-testing biomarker panel which can
simultaneously test for multiple HLA alleles at a low cost (<£20) with
turnaround time of <48h.
Sources to corroborate the impact
Each source listed below provides evidence for the corresponding numbered
claim made in section 4 (details of the impact).
Abacavir
- BHIVA prescribing guidelines for antiretrovirals (2008)
http://www.liv.ac.uk/hiv/BHIVATreatmentGuidelines2008.pdf
- FDA abacavir warning on abacavir hypersensitivity and HLA testing
(2008 onwards)
http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm094302.htm
- EMEA warning for products containing abacavir (2008 onwards) http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000581/human_med_000878.jsp&murl=menus/medicines/medicines.jsp&jsenabled=true
- WHO Collaborating Centre for International Drug Monitoring, Uppsala
Sweden, newsletter (2008) http://www.who.int/medicines/publications/newsletter/2008news3.pdf
- WATERS LJ, MANDALIA S, GAZZARD B, NELSON M. (2007). Prospective
HLA-B*5701 screening and abacavir hypersensitivity: a single centre
experience. AIDS. 21:2533-4 [academic article demonstrating how
screening for abacavir reduces the occurrence of abacavir
hypersensitivity. Although demonstrated in 2007, the impact of the
testing to prevent hypersensitivity continues to the present day].
- Case study on abacavir hypersensitivity and HLA-B*57:01 testing
(including mention of Delphic) in the Academy of Medical Science report
on stratified medicine.
http://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&frm=1&source=web&cd=8&ved=0CFgQFjAH&url=http%3A%2F%2Fwww.acmedsci.ac.uk%2Fdownload.php%3Ffile%3D%2Fimages%2Fproject%2FCasestud.pdf&ei=Q-1NUs3UFeah0QW9oIGgAw&usg=AFQjCNHAl0ubLPdOdLJlfNGSfQQNLCwgzA&sig2=s0w_g6KKm-_hmyiTmeOiFw
(see case study 2)
Carbamazepine
- FDA carbamazepine warning instructing prescribers to test for
HLA-B*15:02 in certain ethnic groups but not in Caucasians (December
2007) (citing [3]) http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124718.htm
- MHRA carbamazepine warning article Drug Safety Update April 2008;
Vol 1, Issue 9: 5:
http://www.mhra.gov.uk/Safetyinformation/DrugSafetyUpdate/CON084888
- Summary of product characteristic (drug label) for carbamazepine with
the warnings about HLA-A*31:01 and risk of carbamazepine
hypersensitivity in Caucasians.
http://www.medicines.org.uk/emc/medicine/24201/SPC/Tegretol+Prolonged+Release+200mg+and+400mg+Tablets+%28formerly+Tegretol+retard%29/