Medical microwave treatments for Menorrhagia and Cancer
Submitting Institution
University of BathUnit of Assessment
PhysicsSummary Impact Type
EconomicResearch Subject Area(s)
Technology: Communications Technologies
Summary of the impact
Microsulis Medical Ltd was founded in 1997 by the University of Bath to
commercialise Professor
Nigel Cronin's invention of a device for microwave endometrial ablation
(MEA) for use in treating
excessive menstrual bleeding (menorrhagia). This minimally invasive
therapy has a success rate
exceeding 80% and remarkably short treatment and recuperation times. It
has been used to treat
over 20,000 patients worldwide since 2008. In Feb 2011 Microsulis sold the
rights to its MEA
device for $3m to a US company in order to concentrate on another
application of Cronin's
microwave technology, namely microwave tissue ablation (MTA) for use in
treating cancer.
Microsulis MTA systems are in place in over 100 hospitals worldwide and
have been used in over
5000 treatments of tumours of the liver, lung, kidney and bone, including
otherwise inoperable
cases. In Feb 2013, the company was bought by AngioDynamics (a major
international provider
of healthcare devices) for $15m. This acquisition is expected to provide a
major boost to both the
reach of the life-saving MTA technology and global sales. Currently
Microsulis employ around 20
people at their base near Portsmouth, producing and developing their MTA
devices. Their sales
revenue since 2008 totals over £11m.
Underpinning research
The fundamental idea of the underlying research is to destroy diseased or
unwanted tissue by
locally heating it using microwaves. There are two key aspects of the
methodology. The first is the
design of the microwave devices. The central challenge here is to deliver
the microwave power to
the tip of the probe, from where it radiates into the surrounding tissue,
without the probe itself
becoming hot. For example, with the percutaneous (through-the-skin)
microwave tissue ablation
(pMTA) device, 180 W of microwave power at 2.45 GHz is delivered to the
tip of a needle-like
probe with a diameter of 1.8 mm. This produces a highly controlled heating
over a large enough
volume to destroy a tumour. To keep the probe cool, water is circulated at
high speeds under 4 bar
of pressure. Cronin (Professor, Department of Physics, 1982-2011), and his
Medical Physics group
(including PhD students Clegg, Hardie, Feldberg) have been at the heart of
this development since
its initiation in 1994. They have designed, built and patented the
microwave probes [1].
The second aspect is to understand the effects of microwave irradiation
on various tissues, for
example, investigating how the heating effects depend on frequency and the
ways in which heat is
dissipated via blood flow, etc. Experimental [2,3] and modelling studies
[4] of these aspects led to
clinical trials [2,5,6]. Cronin and his group have also been heavily
involved in these experimental
and clinical studies. Without their work, the MEA, MTA and pMTA devices
developed and
marketed by Microsulis during the REF period would not exist.
The University of Bath has actively supported Microsulis over a period of
13 years, initially
providing the company with their own laboratory space on campus and by
granting Cronin
substantial periods of secondment to the company to further the
collaboration. Though recently
retired from the University (2011), Cronin remains their Chief Scientist.
His research has also been
supported by substantial contracts with Microsulis [7], and joint grants
between Microsulis and the
University, funded by the Department of Health/EPSRC Health Technology
Devices Programme
[8].
References to the research
[1]. N. Cronin, Microwave applicator, patent num. WO 1999056642
A1, filed May 1999.
[2](*) AD Strickland, PJ Clegg, NJ Cronin, B Swift, M Festing, KP West,
GSM Robertson and DM
Lloyd, Experimental study of large-volume microwave ablation in the
liver, British Journal of
Surgery, 89, 1003-1007 (2002).
doi:10.1046/j.1365-2168.2002.02155.x
[3](*) AU Hines-Peralta, N Pirani, P Clegg, N Cronin, TP Ryan, ZJ Liu and
SN Goldberg,
Microwave ablation: Results with a 2.45 GHz applicator in ex vivo
bovine and in vivo porcine liver,
Radiology, 239, 94-102 (2006). doi:10.1148/radiol.2383050262
[4](*) D Hardie, AJ Sangster and NJ Cronin, Coupled field analysis of
heat flow in the near field of
a microwave applicator for tumour ablation, Electromagnetic Biology
and Medicine, 25, 29-43
(2006). doi:10.1080/15368370600572953
[5]. DA Hodgson, IB Feldberg, N Sharp, N Cronin, M Evans and L
Hirschowitz, Microwave
endometrial ablation: development, clinical trials and outcomes at three
years, British Journal of
Obstetrics and Gynaecology, 106, 684-694 (1999). DOI:
10.1111/j.1471-0528.1999.tb08368.x
[6]. AD. Strickland, PJ. Clegg, NJ. Cronin, Mosheir Elabassy and David M.
Lloyd Rapid
Microwave Ablation of large hepatocellular carcinoma in a high-risk
patient. Asian J Surg. 28, 151
(2005): doi:10.1016/S1015-9584(09)60282-7
[7]. Microwave Ablation of Bone Tumours, Microsulis Medical Ltd,
£871,896. (1/08/2007 -
31/07/2010).
[8]. Microwave Coagulation Therapy for Liver Cancer, EPSRC Grant
GR/R27853 £161,317
(01/09/2001-31/08/2004); Percutaneous Microwave Ablation of Bone
Tumours, Department of
Health, £177,197 (01/11/2009-31/10/2012).
(*) Best indicators of research quality
Details of the impact
Microwave Endometrial Ablation
In 1997, The University of Bath founded Microsulis Medical Ltd to
commercialise Professor Nigel
Cronin's invention of a device for microwave endometrial ablation (MEA).
Endometrial ablation is a
treatment for severe cases of menorrhagia (heavy menstrual bleeding) as an
alternative to
hysterectomy. Following clinical trials
[5], Microsulis' MEA device was
approved by the National Institute for
Clinical Excellence (NICE) [9], the U.S.
Food and Drug Administration (FDA)
[9] and gained the European kite mark.
The device underwent further
development over the past 15 years
and since 2008 has been widely used
to treat menorrhagia.
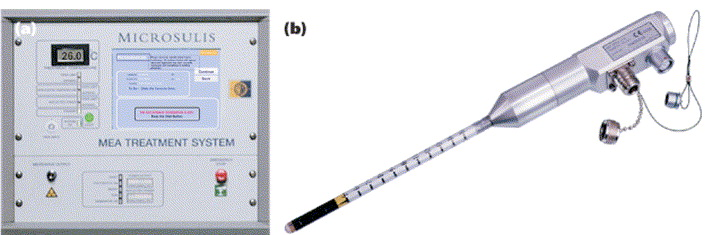 Microsulis MEA system, showing the microwave probe (right)
Microsulis MEA system, showing the microwave probe (right)
In summary the impact from the MEA system is:
- There are over 400 systems in hospitals worldwide and about 20,000
patients have been
treated during the REF period in the UK, USA, Canada, Australia, South
Africa and New
Zealand [10].
- The treatment is fast and safe and can treat all areas of the uterus
[9,11]. It removes the
danger of death (0.1%) [12] associated with hysterectomy.
- A success rate of over 80% in dramatically reducing or stopping
menstrual bleeding
[9,11,13].
- Treatment only takes three minutes and because it is minimally
invasive, patients usually
go home the same day [9]. This compares with the 3-6 days hospital stay
associated with a
typical hysterectomy, followed by recuperation time of anything up to
two months [11,12].
- Accordingly there are huge savings in time for doctors and patients
alike. Additionally,
because the uterus remains intact, there are also major psychological
advantages for the
patient. This all translates into massive cost savings for health care
providers. [9]
In February 2011, Microsulis sold the rights to its MEA device to
Novasure of Massachusetts for
$3M [14]. The sale provided funding for Microsulis to develop
ground-breaking devices for
microwave tissue ablation (MTA) and percutaneous microwave tissue ablation
(pMTA) based on
Cronin's research.
Microwave Tissue Ablation
The idea behind MTA is to use microwaves to produce a highly controlled,
localised and rapid
heating that destroys cancerous tumours. Initial development work
concentrated on liver tumours
[3-6]. Secondary liver tumours are common in cases of bowel cancer and, if
they are controlled,
there is a good chance of preventing further spread of the cancer. An MTA
device for use in open
surgery has been developed and following successful clinical trials [3,6]
obtained CE mark and
United States FDA approval [15]. Since its launch in 2008, over 100 MTA
systems have been
installed in hospitals around the world and nearly 700 treatments have
been completed, with high
success rates [10,16].
A needle-like (percutaneous) MTA device has also been developed and
gained European CE
mark, ETL safety accreditation and United States FDA approval [15] in
2010. The device has the
great advantage of being applied through the skin, rather than requiring
open surgery. This has a
number of advantages for patients, doctors and hospitals alike:
- It gives surgeons the option to treat previously inoperable
patients with tumours in
inaccessible locations [6,17].
- A recent international review of clinical effectiveness [16] found
that of 250 ablated tumours
in 135 patients, local recurrence was observed at only 5% of
ablated sites
- The treatment costs approximately £2,500 per session and it
can be repeated. Surgery costs up to twice this amount and
there is less chance it can be repeated [18].
- The procedure is less invasive than open surgery, meaning less
time in theatre, fewer medical complications and shorter
recuperation times [19].
- Increased patient throughput and reduced operating and patient stay
costs and staff costs, leading to higher revenues in private healthcare
settings.
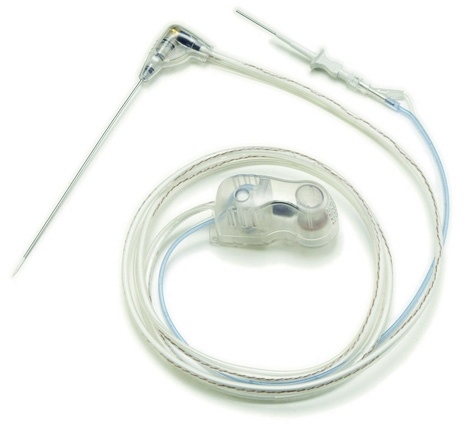 The Microsulis pTMA device
The Microsulis pTMA device
Initial use of the pTMA device has been for liver tumours [16], but lung
[18], kidney, pancreas and
bone treatments are being performed [10]. Although only released in late
2010, pMTA is already in
use at over 80 surgical and radiological centres [10]. Over 5000 patients
have been treated with
MTA/pTMA since 2008 and the number of new treatments is more than doubling
year on year [10].
Whilst there are many competing companies and devices in the oncology
arena, Microsulis are
well ahead of their competitors in terms of device performance and ease of
use. Commenting on
the Microsulis system, the consultant radiologist at Oxford University
Hospitals Trust states "This is
a fantastic development. It will kill small tumours in minutes and we are
examining how it can
improve survival by reducing cancer mass in larger tumours..... We believe
it could save -
thousands of lives a year." [18]
Microsulis employ around 20 people at their Portsmouth base, from where
they produce and sell
their third-generation MTA and pMTA devices [10] (jobs in the medical
instruments sector carry a
high output multiplier of ≈2.5 [20]). They have recently invested £250k in
a new in-house
production and packaging facility, increasing its manufacturing capacity.
Microsulis's sales revenue
in the REF period totals over £11m [10]. The company was named `Innovator
of the Year' at The
News Business Excellence Awards in 2012 and also claimed the runner up
prize in the `Exporter of
the Year' category [14].
In February 2013, Microsulis was acquired by AngioDynamics of Albany (New
York) — a leading
international provider of medical devices. The sale for $15m [21] is
expected to provide new
impetus for global sales of the pMTA device (which is being marketed as
"Acculis MTA"), and
further improve patient access to this lifesaving technology.
In summary, Professor Cronin's invention of new technologies for medical
treatments has
revolutionised healthcare outcomes for tens of thousands of patients and
created substantial
economic impact both directly via the turnover of Microsulis Ltd and
indirectly via savings to
healthcare providers.
Sources to corroborate the impact
[9] FDA:
http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm082313.htm;
NICE: http://guidance.nice.org.uk/TA78
;
(accessed 16/4/13)
[10] Data supplied by Chief Executive Officer, Microsulis Medical Ltd. http://www.microsulis.com.
Note that following their recent acquisition by Angiodynamics the
Microsulis website may be
subject to change.
[11] Microwave Endometrial ablation: http://publications.nice.org.uk/microwave-endometrial-ablation-ipg7/the-procedure
— indications (accessed 22/4/13)
[12] Encyclopedia of Surgery http://www.surgeryencyclopedia.com/Fi-La/Hysterectomy.html
(accessed 16/4/13).
[13] J.M. Cooper, et al, J. Am. Assoc. Gynecol Laparosc 11(3) 394 (2004).
[14] http://www.businesswire.com/news/home/20110204005902/en/Microsulis-sells-Microwave-Endometrial-Ablation-MEA-Hologic
[15] For FDA approval see: http://www.fda.gov/
(accessed 16/4/2013)..
[16] D.M. Lloyd et al, HPB 13 579 (2011). doi:
10.1111/j.1477-2574.2011.00338.x.
[17] N. Bhardwaj, A.D. Strickland, F. Ahmad, M. El-Abassy, B. Morgan,
G.S.M. Robertson, D.M.
Lloyd, European Journal of Surgical Oncology 36, 264-268 (2010)
http://dx.doi.org/10.1016/j.ejso.2009.10.006
[18] Consultant radiologist quoted in Sunday Express newspaper article: "It's
fantastic. Docs
cooked my Cancer" , Nov 27, 2011. http://www.express.co.uk/news/uk/286269/It-s-fantastic-Docs-cooked-my-cancer
(accessed 16/4/13)
[19] C. Jones, S.A. Badger, G. Ellis, The Surgeon, 9, 33 (2011).
[20] The Economic Impact of UK higher education institutions,
report by Universities UK. (2007).
http://www.universitiesuk.ac.uk/highereducation/Pages/EconomicImpact3.aspx
(accessed
15/4/2013)
[21] http://www.bizjournals.com/albany/news/2013/02/01/angiodynamics-drops-15m-on-another.html
(accessed 22/7/13).