CHEM09 - Short and long-acting insulins for the management of diabetes
Submitting Institution
University of YorkUnit of Assessment
ChemistrySummary Impact Type
HealthResearch Subject Area(s)
Biological Sciences: Biochemistry and Cell Biology
Medical and Health Sciences: Clinical Sciences, Pharmacology and Pharmaceutical Sciences
Summary of the impact
Insulin derivatives that stem directly from structural work carried out
within the York Structural Biology Laboratory (YSBL) are now the standard
treatment for insulin-dependent diabetes for some 35 million patients
worldwide. The successful development of new insulin drugs hinged upon
controlling their speed of action following intravenous administration.
This speed of action is controlled by insulin's degree of aggregation,
which, in turn, is determined by protein-protein interactions.
Understanding, modifying and controlling these interactions depended on
detailed structural studies of insulin, insulin mutants and insulin
derivatives. The most widely used derivatives were developed following
structural work carried out within YSBL in the Department of Chemistry.
The research has had economic impact through sales of the insulin drugs
(over $6 billion in 2012) and major health impacts on diabetics worldwide.
Underpinning research
Background to research. The late GG Dodson FRS played a major role
in the initial determination and analysis of the structure of insulin by
X-ray crystallography in the Oxford laboratory of Nobel laureate DC
Hodgkin. The insulin project moved to York when Hodgkin retired, and over
the next twenty years many further structures of native insulin, insulin
mutants and insulin derivatives were determined. These structures allowed
both academic and industrial scientists to understand how the structure of
insulin relates to its biological activity, and how the physico-chemical
properties of different preparations of insulin can be engineered to
provide therapeutic options.
Naturally occurring insulin is stored in the pancreas in crystals, made
up of three insulin dimers coordinated by zinc ions. They dissociate into
active insulin monomer upon release into the blood stream. A basal level
of insulin (pM) is maintained in the blood stream and increases in
response to increased glucose in the blood following a meal. The original
therapy for Type I diabetics was injection of insulin crystals extracted
from pig or cow pancreas. However, it did not reproduce the physiological
blood levels of insulin and caused immune responses to foreign hormones.
All these led to the long-term complications associated with insulin
therapy.
From 1984 to 2000, YSBL engaged in a major collaboration on recombinant,
novel modified insulins with Novo Nordisk A/S (www.novonordisk.com).
The structures of these analogues provided a detailed understanding of the
nature of insulin aggregation. The focus of the work in 1993 moved towards
the rational design of modified insulins in order to obtain clinically
applicable monomeric insulins — they are now the basis of the modern
"fast-acting" insulins. Furthermore, structures of insulin crystals with
various additives and/or modifications identified some general principles
on how to increase stability of insulin hexamers, leading to the current
"long-lasting" insulin preparations.
Research during period. The key breakthroughs during the
assessment period were carried out by J. L Whittingham and R. E. Hubbard
with G. G. Dodson:
-
Design of rapid-acting monomeric insulins: The crystal
structure of insulin hexamers and dimers suggested amino acid mutations,
which would lead to a monomeric insulin. The structures of such proteins
are reported in references 1 (1993) and 2 (1998). In one of these
proteins, B28 proline is mutated to aspartic acid; this is the insulin
of the Novo Nordisk product Insulin aspart.®
-
Identifying additives to stabilise insulin preparations:
References 2 (1998) and 3 (1995) describe structures of series of
preparations in which hexamer formation is stabilised. The structure of
a m-cresol-insulin clathrate led to the proposal that mutation
to tryptophan at the end of the B chain would disrupt the insulin
aggregate. This mutant was found to have improved stability in Novo
Nordisk's very complex protein manufacturing processes.
-
Modifications to generate long-active insulins: References 4
(1997) and 5 (2004) report the crystal structures of acylated insulins
which had been identified by Novo Nordisk as prolonged-acting insulins.
The YSBL research suggested that the prolonged action was more likely to
be related to the way that these modified insulins aggregated in
solution (and in the crystals). These soluble prolonged-acting insulins
are significantly better for diabetics than other long-acting insulins.
Insulin research in York continues. Throughout the 2000s AM Brzozowski of
YSBL worked with an international team on the development of super-active
analogues of insulin. These have provided the tools that enabled a
breakthrough in 2013 (reference 6) in the determination of the structure
of the first insulin receptor-insulin complex. Insights from this work
will have an impact in the future on the design of further insulin
analogues with the prospect of oral delivery of insulin.
Key researchers:
G. Guy Dodson FRS, appointed 01/07/1976 as Lecturer, Professor from
01/08/1993, Emeritus Professor from 01/08/2005. Died 2012.
Roderick E. Hubbard, appointed 01/10/1980 as temporary Lecturer,
Professor from 01/10/1995
A. Marek Brzozowski, appointed 06/04/1989 as Research Fellow, Reader
01/10/2002
Jean L. Whittingham, appointed 01/01/95 as Research Fellow
References to the research
This research exceeds the quality threshold as is evident from the
journal quality and the number of citations. Citations from Scopus
(20/09/2013). Refereed publications (authors in publications 1-5 are
either from YSBL or YSBL and Novo-Nordisk).
1. E. J. Dodson, G. G. Dodson, R. E. Hubbard, P. C. E. Moody, J.
Turkenburg, J. L. Whittingham, B. Xiao, J. Brange, Kaarsholm and H.
Thogersen, H. "Insulin assembly: its modification by protein engineering
and ligand binding", Phil. Trans. R. Soc. Lond. A, 1993, 345,
153-164. DOI: 10.1098/rsta.1993.0126. 14 citations.
2. J. L. Whittingham, D. J. Edwards, A. A. Antson, J. M. Clarkson and G.
G. Dodson, "Interactions of Phenol and m-Cresol in the Insulin Hexamer,
and Their Effect on the Association Properties of B28 Pro → Asp Insulin
Analogues", Biochemistry, 1998, 37, 11516-11523. DOI:
10.1021/bi980807s. 59 citations.
3. J. L. Whittingham, S. Chaudhuri, E. J. Dodson, P. C. E Moody and G. G.
Dodson. "X-ray Crystallographic Studies on Hexameric Insulins in the
Presence of Helix-stabilising Agents, Thiocyanate, Methylparaben, and
Phenol", Biochemistry, 1995, 34, 15553-15563. DOI:
10.1021/bi00047a022. 69 citations.
4. J. L. Whittingham, S. Havelund and I. Jonassen, "Crystal Structure of
a Prolonged-Acting Insulin with Albumin-Binding Properties", Biochemistry,
1997, 36, 2826-2831. DOI: 10.1021/bi9625105. 77 citations.
5. J. L. Whittingham, I. Jonassen, S. Havelund, S. M. Roberts, E. J.
Dodson, C.S. Verma, A. J. Wilkinson and G. G. Dodson, "Crystallographic
and Solution Studies of N-Lithocholyl Insulin: A New Generation of
Prolonged-Acting Human Insulins", Biochemistry, 2004, 43,
5987-5995. DOI: 10.1021/bi036163s. 22 citations.
6. J. G. Menting, J. Whittaker, M. B. Margetts, L. J. Whittaker, G. K.-W.
Kong, B. J. Smith, C. W. Watson, L. Žáková, E. Kletvíková, J. Jiráček, D.
F. Steiner, S. J. Chan, G. G. Dodson, A. M. Brzozowski, M. W. Weiss, C. W.
Ward and M. C. Lawrence. "How insulin engages its primary binding site on
the insulin receptor", Nature, 2013, 493, 241-245. DOI:
10.1038/nature11781. 11 citations. Patent PUV 2012-26680.
Details of the impact
Worldwide there are some 35 million sufferers of insulin-dependent
diabetes. For many years, the adverse symptoms of this condition have been
mitigated successfully with regular injections of insulin. Initially these
insulins were obtained from bovine and/or porcine sources, but they did
not mimic well actions of endogenous insulin, leading to frequent
hypo-glycaemia and other complications. In 1980, the first clinical trials
were begun of recombinant insulins obtained by protein engineering
methods. From these trials it soon became apparent that the rate of
insulin action in vivo was a key factor in the therapeutic
potential of the enzyme. The best results were obtained when the insulin
injectate contained a mixture of fast-acting and slow-acting insulins,
where the latter avoids the need to have regular repeated injections and
minimise hypoglycemia, and the former is necessary to treat the rapid rise
in blood glucose levels that accompanies ingestion of food.
Accordingly recombinant insulin producers concentrated on controlling the
rate of action of insulin and its analogues. Amongst these, Novo-Nordisk
in collaboration with YSBL, led the way in trying to find a structural
rationale for the controlled disaggregation rates of hexameric insulin.
YSBL was the world's leading laboratory for insulin structures, and the
large majority of the structural work for Novo-Nordisk on insulins from
1993 to 2000 was done in conjunction with YSBL. The protein structures
published from York are available as coordinates in the Protein Data Bank.7
The collaboration between YSBL and Novo-Nordisk was supported by several
grants.8 This development programme was highly successful and
consequently today Novo-Nordisk is the leading developer and world's
largest producer of recombinant insulin for treatment of diabetes.
The first new product that came from York structural studies was the
aspartate mutant described above.1,2 This fast-acting insulin
was launched in 1999, marketed as NovoLog® in the US and
Insulin Novorapid® in Europe.9
The second development came from the derivatised insulins, which were
conceived directly from the structural work described in reference 4
above. It was discovered that the attachment of a fatty acid to insulin
led to its prolonged action, resulting in just one daily intravenous
administration of the hormone. These derivatised insulins received FDA
approval in 2005, and are marketed as the product Levemir® (or
Insulin Detemir®) and are now the mainstay of Novo Nordisk's
insulin products. Its new, improved generation insulin Degludec®
was approved by the EU and Chuikyo (Japan) in 2013.
Levemir®, NovoRapid and NovoLog® (also marketed as
Novomix® in a different formulation) are true blockbuster drugs
with billion dollar sales (reference 10). Levemir® had US sales
of $1.7 billion in 2012 growing from $756 million in 2008. The
corresponding figures for Novolog® are $1.6 billion and $1.1
billion, and for Novorapid, $2.7 billion and $1.5 billion. Levemir®
and Novolog® are currently number 40 and 42 in the ranking of
US drugs by sales. Total worldwide sales of the drugs were $6 billion in
2012 (Figure).10
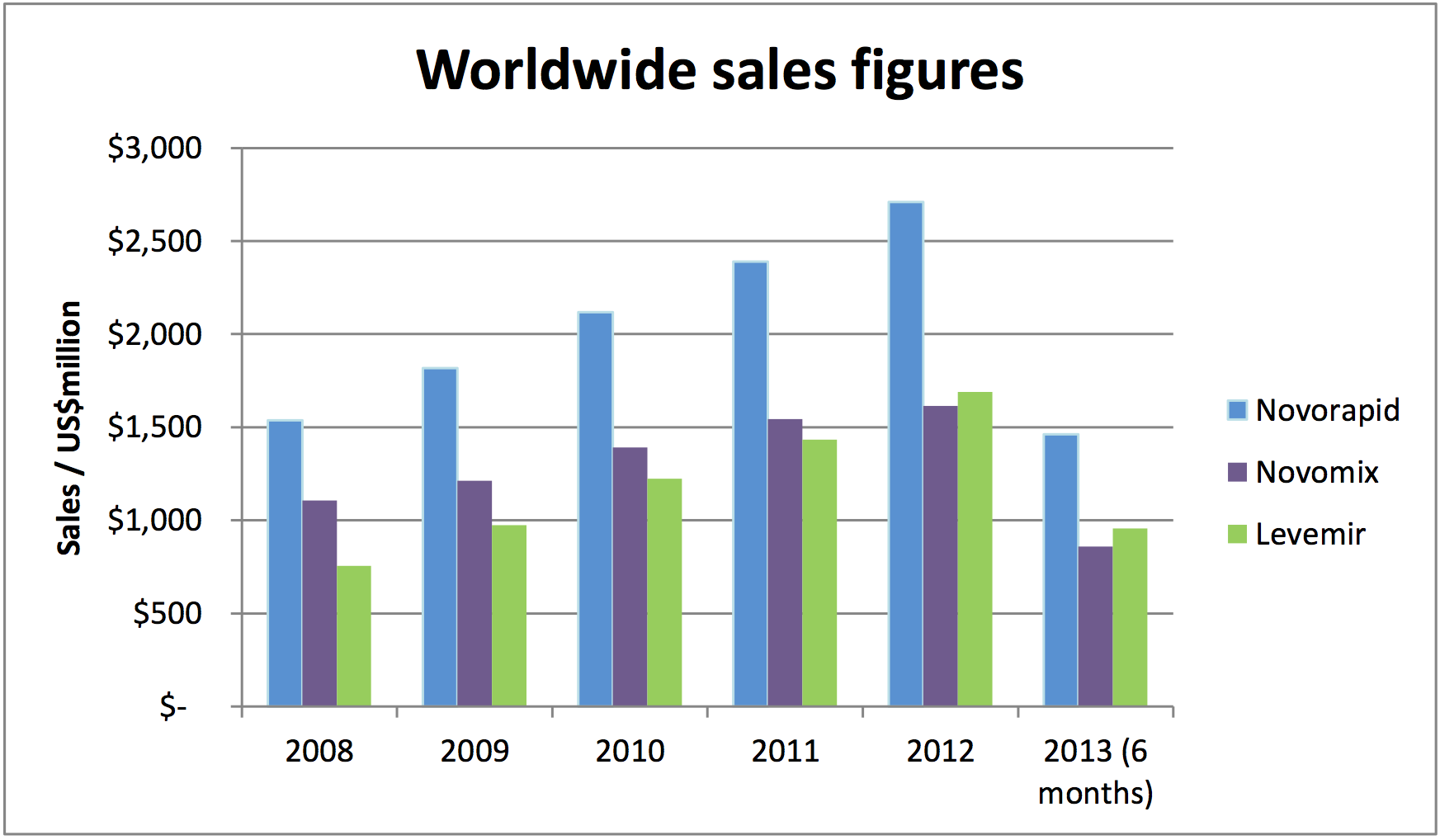 Figure: Worldwide sales of Novorapid, Levemir® and Novomix® (alternative name for Novolog® depending on market), during REF impact period.10
Figure: Worldwide sales of Novorapid, Levemir® and Novomix® (alternative name for Novolog® depending on market), during REF impact period.10
Svend Ludvigsen (Vice-President, Diabetes formulation, biophysics and
structure) at Novo Nordisk assesses the impact of York's work as follows:11
"Throughout the years the collaboration with York has been a continuous
source of inspiration for the understanding of insulin structure and
insulin as pharmaceutical products. The work of Whittingham et al. 1997
has provided significant insight into some of the protraction principles
of the insulin analog, insulin Detemir, developed into a once daily basal
insulin product Levemir®. Novorapid® (US: Novolog®) and Levemir® both have
blockbuster status and are used by millions of patients all around the
globe."
Sources to corroborate the impact
- Deposition of coordinates on Protein Data Bank (PDB). http://www.rcsb.org.
22 insulin structures deposited with Whittingham as co-author (1995
onwards) such as entries — 1UZ9, 1ZEG, 3ZU1, 1MPJ
- Seven successive, uninterrupted, grant renewals from Novozymes and
Novo-Nordisk since 1993- 2013, totalling more than £3.4M.
- NovoLog® and Novorapid® details: www.ukmi.nhs.uk/NewMaterial/html/docs/insulin.pdf
and http://www.globalrph.com/rapid-acting-analogues.htm
- Sales of insulins from Novo-Nordisk website: www.novonordisk.com
e.g. http://www.novonordisk.com/images/investors/investor_presentations/2013/Interim_report/PR130808_H1_results_UK.pdf
- Vice President, Diabetes formulation, structure and biophysics,
Novo-Nordisk A/S